Fluoroacetic acid occurs in gifblaar, one of the most poisonous of all plants. A 0.318 M solution
Question:
Fluoroacetic acid occurs in gifblaar, one of the most poisonous of all plants. A 0.318 M solution of the acid is found to have a pH = 1.56. Calculate Ka of fluoroacetic acid.
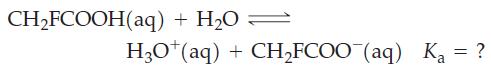
Transcribed Image Text:
CH₂FCOOH(aq) + H₂O = H3O+ (aq) + CH₂FCOO (aq) Ka = ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
To calculate the Ka of fluoroacetic acid from the given inf...View the full answer

Answered By

WAHIDUL HAQUE
hello,
I'm a professional academic solution provider working as a freelance academic solution provider since 7 years. I have completed numerous projects. Help lots of students to get good marks in their exams and quizzes. I can provide any type of academic help to your homework, classwork etc, if you are a student of Accounting, Finance, Economics, Statistics. I believe in satisfying client by my work quality, rather than making one-time profit. I charge reasonable so that we make good long term relationship. why will you choose me? i am an extremely passionate, boldly honest, ethically driven and pro-active contractor that holds each of my clients in high regards throughout all my business relations. in addition, I'll always make sure that I'm giving my 100% better in every work that will be entrusted to me to be able to produce an outcome that will meet my client's standards. so if you are a student that is now reading my profile and considering me for your academic help. please feel free to look through my working history, feedback and contact me if you see or read something that interests you. I appreciate your time and consideration.
regards
4.90+
233+ Reviews
368+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
Googles ease of use and superior search results have propelled the search engine to its num- ber one status, ousting the early dominance of competitors such as WebCrawler and Infos- eek. Even later...
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
This case will enable you to practice conducting planning and substantive analytical procedures for accounts in the revenue cycle. When analyzing the financial data, you may assume that the 2015...
-
Springer Products wishes to borrow $80,000 from a local bank using its accounts receivable to secure the loan. The bank's policy is to accept as collateral any accounts that are normally paid within...
-
Exercise 5 gave the following data on weekly gross revenue, television advertising, and newspaper advertising for Showtime Movie Theaters. a. Find an estimated regression equation relating weekly...
-
4. Consumer Products Co reports growth by segment in the Managements Discussion and Analysis section of its annual report (shown here in Exhibit 15.6, Part A) for a given year. For this year, does...
-
Capital budgeting has the same focus as accrual accounting. Do you agree? Explain.
-
can anyone help me Problem III - Notes Receivable (20 points) On December 31, 2019. Arden Corporation rendered services to Fly Company, accepting 580.000 immediate cash and agreeing to accept the...
-
Caproic acid, HC 6 H 11 O 2 , found in small amounts in coconut and palm oils, is used in making artificial flavors. A saturated aqueous solution of the acid contains 11 g/L and has pH = 2.94....
-
A 625 mL sample of an aqueous solution containing 0.275 mol propionic acid, CH 3 CH 2 CO 2 H, has [H 3 O + ] = 0.00239 M. What is the value of K a for propionic acid? CH3CHCOH + H0 H3O+ + CH3CHCO Ka...
-
Why is government spending (G) an injection? Why are taxes (T) a leakage?
-
Listed in the accompanying table are waiting times (seconds) of observed cars at a Delaware inspection station. The data from two waiting lines are real observations, and the data from the sir line...
-
Franklin Prepared Foods (FPF) sells three varieties of microwaveable meals with the following prices and costs: Variable Cost Fixed Cost per Meat Fish Vegetarian Entire firm Selling Price per Case: $...
-
Isabella is a 14-year-old Hispanic bisexual female who has come into the Department of Child Safety (DCS) care due to neglect. Isabella's mother, Martina, is 35 years old, a single mother, has an...
-
Jeff is able to ride a bicycle although he hasn't ridden one for a few years, thanks to his: ( A ) procedural memory ( B ) episodic memory C ) semantic memory ( D ) cognitive memory
-
1. Allen Young has always been proud of his personal investment strategies and has done very well over the past several years. He invests primarily in the stock market. Over the past several months,...
-
Joe, a student, asks your help in understanding some characteristics of a corporation. Explain each of these to Joe. (a) Separate legal existence. (b) Limited liability of stockholders. (c)...
-
Kenneth Hubbard has prepared the following list of statements about managerial accounting and financial accounting. 1. Financial accounting focuses on providing information to internal users. 2....
-
If the effect of the debit portion of an adjusting entry is to increase the balance of an asset account, which of the following statements describes the effect of the credit portion of the entry? (a)...
-
If the effect of the credit portion of an adjusting entry is to increase the balance of a liability account, which of the following statements describes the effect of the debit portion of the entry?...
-
Does every adjusting entry have an effect on determining the amount of net income for a period? Explain.
-
Provide a graph chart or data with sample numbers indicating Valuing Stocks and Bonds?
-
I just need help with part b. It says that the answer is not complete and some are wrong. So can you kindly fix it for me and give me the full answers as it says the answer is "not complete". Thank...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...

Study smarter with the SolutionInn App


