For a solution containing 6.38% para-dichlorobenzene by mass in benzene, the density of the solution as a
Question:
For a solution containing 6.38% para-dichlorobenzene by mass in benzene, the density of the solution as a function of temperature (t) in the temperature range 15 to 65 °C is given by the equation
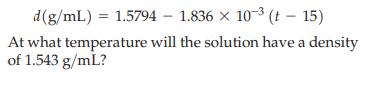
Transcribed Image Text:
d(g/mL) = 1.5794 1.836 × 10-³ (15) X At what temperature will the solution have a density of 1.543 g/mL?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
To find the temperature at which the solution will have a density of 1543 gmL we ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The conversion of the kinetic energy of wind to electricity may be an attractive alternative to the use of fossil fuels. Typically, wind causes the rotor of a turbine to turn, and a generator...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
Hi, the task is to critically evaluated two or more types of market segmentation, and applied to own organisations customer base. The guidance says to start with a general explanation of the topic -...
-
Explain what is meant by agency relationships and agency costs.
-
1. How would you describe the founding team of Fenton, Hoffer, and Le Tuan? Is it a balanced team? What does each member bring to the business? Can you see gaps in their skills and capabilities that...
-
What is lot traceability? Why is it important to safety related products?
-
This problem continues the Davis Consulting situation from Problem P10-23. Davis Consulting believes the company will need to borrow $ 300,000 in order to expand operations. Davis consults the bank...
-
Information is given about a company's production of a product. Target Costing: estimated units sold: 500,000 Market Price: $15.00 Investment: $300,000.00 Return of Investment: 15% Questions: 1....
-
According to the rules on significant figures, the product of the measured quantities 99.9 m and 1.008 m should be expressed to three significant figures101 m 2 . Yet, in this case, it would be more...
-
A solution containing 12.0% sodium hydroxide by mass in water has a density of 1.131 g/mL. What volume of this solution, in liters, must be used in an application requiring 2.75 kg of sodium...
-
Explain skills in mediation, which aims to resolve disagreements between individuals? LOP1
-
In this problem, we consider mild modifications of the standard MDP setting. (a) (10 points) Sometimes MDPs are formulated with a reward function R(s) that depends only on the current state. Write...
-
All-Walnut, Inc. produces two models of bookcases. The bookcases sell for the amount listed in the table below. Each bookcase requires a certain number of labor hours, machine time, and materials...
-
Problem 1 Find the number of degrees of freedom of the mechanisms (a)-(d) (a) (b)
-
3) (10 pts) The following grammar is given E EAE (E) -E | id V={E,A), T={-,(,),*,/,+,id} and starting symbol is E. a) Give the left-most derivation of w= id+id*id. Is w accepted? b) Is this a...
-
4. X, the proprietor of a departmental store, decided to calculate separate profits for his two departments L and M for the month ending 31st January. Stock on 31st January could not be valued for...
-
The hydrolysis of glycinamide is catalyzed by [Co(ethylenediamine)2]2+ Propose a mechanism for this reaction. Co2+ H2NCH2CNH2 H20
-
The graph of an equation is given. (a) Find the intercepts. (b) Indicate whether the graph is symmetric with respect to the x-axis, the y-axis, or the origin. -3 6 -6 3 x
-
The cash price of a machine tool is $3500. The dealer is willing to accept a $1200 down payment and 24 end-of-month monthly payments of $110 each. At what effective interest rate are these terms...
-
A local bank makes automobile loans. It charges 4% per year in the following manner: if$3600 is borrowed to be repaid over a 3-year period, the bank interest charge is $3600 x 0.04 x 3 years = $432....
-
Upon graduation, every engineer must decide whether to go on to graduate school. Estimate the costs of going full time to the university to obtain a Master of Science degree. Then estimate the...
-
06:27 82% . . 4 Document (1) Question Management accounting has evolved over the years to provide information for: determining the cost of product and services and financial control; management...
-
R paid the following state and local taxes during the current year: State income taxes due with his prior year tax return $ 700 State income taxes withheld by his employer 2,500 Real estate taxes...
-
View Policies Current Attempt in Progress Blossom Company owns equipment that cost $ 62, 100 when purchased on January 1, 2019. It has been depreciated using the straight- line method based on...

Study smarter with the SolutionInn App


