For the following equilibrium reactions, calculate r G at the indicated temperature. (a) H(g) + I2(g)
Question:
For the following equilibrium reactions, calculate ΔrG° at the indicated temperature.
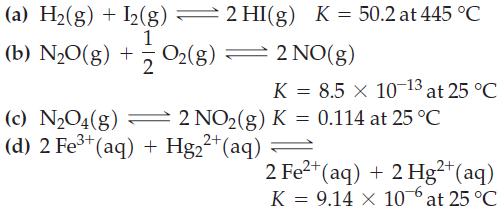
Transcribed Image Text:
(a) H₂(g) + I2(g) 2 HI(g) K = 50.2 at 445 °C 1 (b) N₂0(g) + O2(g) 2 = 2 NO(g) K = 8.5 × 10-13 at 25 °C (c) N₂O4(g) 2 NO₂(g) K = 0.114 at 25 °C (d) 2 Fe³+ (aq) + Hg₂²+ (aq) 2+ 2 Fe²+ (aq) + 2 Hg²+ (aq) K = 9.14 X 10 at 25 °C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
From the Gibbs free energy equation Delta G 2303RTlogK T temperature in kelvin R 8314 jmole K equi...View the full answer

Answered By

Ehsan Mahmood
I’ve earned Masters Degree in Business Studies and specialized in Accounts & Finance. Couple with this, I have earned BS Sociology from renowned institute of Pakistan. Moreover, I have humongous teaching experience at Graduate and Post-graduate level to Business and humanities students along with more than 7 years of teaching experience to my foreign students Online. I’m also professional writer and write for numerous academic journals pertaining to educational institutes periodically.
4.90+
248+ Reviews
287+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
At a certain temperature the following reactions have the constants shown: Calculate the equilibrium constant Kc for the following reaction at that temperature: S(s) O2(8O2() 4.2 x 102 25(s) + 302(g)...
-
Calculate the equilibrium constant for the acid-base reactions between the following pairs of reactants. a. HCl + H2O b. CH3COOH + H2O c. CH3NH2 + H2O CH3NH3 + H20
-
Calculate the equilibrium constants of the following reactions at 25C from standard potential data: (a) Sn(s) + CuS04 (aq) ~ Cu(s) + SnS04 (aq) (b) Cu2+(aq) + Cu(s) ~ 2 Cu+{aq)
-
Prepare journal entries to record each of the following sales transactions of TFC Merchandising. TFC uses a perpetual inventory system and the gross method. May 1 9 Sold merchandise for $600, with...
-
Danner Company expects to have a cash balance of $45,000 on January 1, 2014. Relevant monthly budget data for the first 2 months of 2014 are as follows. Collections from customers: January $85,000,...
-
A concave mirror of focal length forms an image of the moon. Where is the image located? A. At the mirror's surface B. Almost exactly a distance behind the mirror C. Almost exactly a distance in...
-
What is context-based reasoning?
-
Marilyn County operates on a calendar year basis. It uses a Capital Projects Fund to account for major capital projects and a Debt Service Fund to accumulate resources to pay principal and interest...
-
[The following information applies to the questions displayed below.) Pete's Tennis Shop has the following transactions related to its top-selling Wilson tennis racket for the month of August. Pete's...
-
For the reaction 2 SO 2 (g) + O 2 (g) 2 SO 3 (g), Kc = 2.8 x 10 2 M -1 at 1000 K. (a) What is r G at 1000 K? (b) If 0.40 mol SO 2 0.18 mol O 2 , and 0.72 mol SO 3 are mixed in a 2.50 L flask at...
-
At 1000 K, an equilibrium mixture in the reaction CO 2 (g) + H 2 (g) CO(g) + H 2 O(g) contains 0.276 mol H 2 0.276 mol CO 2 , 0.224 mol CO, and 0.224 mol H 2 O. (a) What is K at 1000 K? (b)...
-
Read the 2014 GLOBE CEO Study (https://globeproject.com/ study_2014). Select three countries and write a two- to three-page paper on the differences in their cultural perspectives on leadership.
-
Show how the buying process occurs in the consumer. Review some of the steps in the buying process, stories like: felt need pre-purchase activity purchase decision Post-purchase feelings Explain and...
-
How did Henry Ford set the stage for some of the same problems we still face today in employee relations, especially in manufacturing? 2) If you were a human resources manager, how would you address...
-
What does a DMO risk by not having a positioning theme? Critique the potential of your destination's slogan to effectively differentiate against rivals. you have been asked by a television network to...
-
Vaporization of mixtures of hexane and octane. Using the T-x-y diagram (Figure 1) on the next page, determine the temperature, amounts, and compositions of the vapor and liquid phases at 1 atm for...
-
what should p&g do to replace lafley when he retires a second time? what actions should they take to prepare for the succession?
-
Two linear transformers are cascaded as shown in Fig. 13.101. Show that o* R(L; + LL - M L +LL-LM; - LM o*(L,L, + L - M})- jaR(L, +L,) Iz. in
-
In the busy port of Chennai, India, the number of containers loaded onto ships during a 15-week period is as follows: 1. Develop a linear trend equation to forecast container loadings. 2. Using the...
-
Standard-costing method, assigning costs. Refer to the information in Exercise 17-24. Suppose Bio Dec determines standard costs of $6.60 per equivalent unit for direct materials and $10.40 per...
-
Transferred-in costs, weighted-average method. Asaya Clothing, Inc. is a manufacturer of winter clothes, It has a Knitting Department and a Finishing Department This exercise focuses on the Finishing...
-
Transferred-in costs, FIFO method. Refer to the information in Exercise 17-27. Suppose that Asaya uses the FIFO method instead of the weighted-average method in all of its departments. The only...
-
1) A portfolio consists of 3 securities have the following characteristics in terms of standard deviation, proportion of investment and correlation coefficient. Security Standard deviation...
-
Find the future values of the ordinary annuities at the given annual rate r compounded as indicated. The payments are made to coincide with the periods of compounding. (Round your answer to the...
-
Your company has preferred stock currently selling for $65.24 on the New York Stock Exchange (NYSE). If the stock paid a dividend of $4.8 last year, what is the cost of preferred stock to your...

Study smarter with the SolutionInn App


