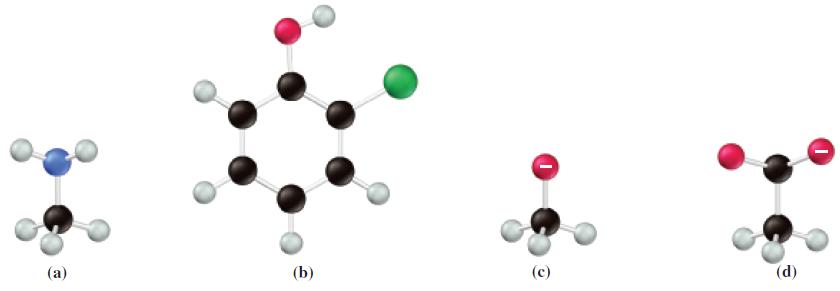
For the molecular models shown, write the formula of the species that is the most acidic and
Question:
For the molecular models shown, write the formula of the species that is the most acidic and the one that is most basic, and give reasons for your choices.
Transcribed Image Text:
(a) (b) (c) (d)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
The most acidic and the most basic species in the image are both aniline Aniline has the chemical fo...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Tom Regan argues that the basic similarity between human beings, and the basic similarity between humans and the animals they eat and trap is that Group of answer choices
-
Consider the molecular models shown here, where X represents a halogen atom. (a) If X is the same atom in both molecules, which one will be more acidic? (b) Does the acidity of each molecule increase...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Engineers observe that about 90% of graphite samples fracture within five hours when subjected to a certain stress. (a) If the time to fracture is modeled with an exponential distribution, what would...
-
Briefly describe each of the following motives for merging: (a) Growth or diversification, (b) Synergy, (c) Fund raising, (d) Increased managerial skill or technology, (e) Tax considerations, (f)...
-
In San Francisco, 30% of workers take public transportation daily (USA Today, December 21, 2005). a. In a sample of 10 workers, what is the probability that exactly three workers take public...
-
What is the purpose of the final project report? AppendixLO1
-
The following numbered items 1 10 state procedures accountants should consider in review engagements and compilation engagements on the annual financial statements of non-issuers (performed in...
-
Why is the R-squared of the portfolio not the same as the average of all stock R-squares?
-
For each reaction draw a Lewis structure for each species and indicate which is the acid and which is the base: (a) CO + HO HCO3 HOBF3 (b) HO + BF3 (c) 0 + HO 2OH- (d) S + SO3 SO3-
-
Indicate which of the following is the weakest acid, and give reasons for your choice: HBr; CH 2 ClCOOH; CH 3 CH 2 COOH; CH 2 FCH 2 COOH; CI 3 COOH.
-
Refer to the clustering problem involving the file FBS described in Problem 1. The NCAA has a preference for conferences consisting of similar schools with respect to their endowment, enrollment, and...
-
Discuss the Competitive Markets and Externalities simulations (both with and without policy interventions) . What impact do policy interventions have on the supply and demand equilibrium for a...
-
The best consultant to fix issue number one is Frederick Taylor who is credited with creating the scientific management movement (Lumen, n.d.). Since Taylor's work focused on how a process could be...
-
1. Which Pepsico products are growing faster than soft drinks (why) and by what percentage? 2. Why do the fastest growing products experience a more complex supply chain? Explain. 3. What are some of...
-
Use BLUF (Bottom Line UP Front) or Brief for answering the following questions: 1) There are a number of InfoSec frameworks / models available in industry. A. What is an InfoSec framework / model? B....
-
An introduction to organizational structure. Topics such as alternative organizational structures, the reciprocal relationship between multinational strategy and structure, and how recourses affect...
-
Karen Noonan opened Clean Sweep Inc. on February 1, 2017. During February, the following transactions were completed. Feb. 1 Issued 5,000 shares of Clean Sweep common stock for $13,000. Each share...
-
Marc Company assembles products from a group of interconnecting parts. The company produces some of the parts and buys some from outside vendors. The vendor for Part X has just increased its price by...
-
The accounting firm of Deloitte & Touche is the largest international accounting firm in the world as ranked by total revenues. For the last two years, Deloitte & Touche reported the following for...
-
Office-Brite Cleaning Services, LLC, provides cleaning services for office buildings. The firm has 10 members in the LLC, which did not change between 2008 and 2009. During 2009, the business...
-
What is the objective of most businesses? Explain
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...
-
An investment will pay you $34,000 in 11 years. If the appropriate discount rate is 6.1 percent compounded daily, what is the present value? (Use 365 days a year. Do not round intermediate...

Study smarter with the SolutionInn App


