For the reaction below, r G = 27.07 kJ mol -1 at 298 K. Use this
Question:
For the reaction below, ΔrG° = 27.07 kJ mol-1 at 298 K.
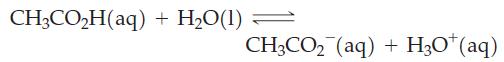 Use this thermodynamic quantity to decide in which direction the reaction is spontaneous when the concentrations of CH3CO2H(aq), CH3CO2-(aq), and H3O+(aq) are 0.10 M, 1.0 x 10-3 M, and 1.0 x 10-3 M, respectively.
Use this thermodynamic quantity to decide in which direction the reaction is spontaneous when the concentrations of CH3CO2H(aq), CH3CO2-(aq), and H3O+(aq) are 0.10 M, 1.0 x 10-3 M, and 1.0 x 10-3 M, respectively.
Transcribed Image Text:
CH3CO₂H(aq) + H₂O(1) CH3CO₂ (aq) + H₂O+ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The standard Gibbs free energy change rG for the reaction CH3COHaq H2O1 CH3CO2 aq H3O aq is 2707 kJ ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
For the reaction below, r G = 29.05 kJ mol -1 at 298 K. Use this thermodynamic quantity to decide in which direction the reaction is spontaneous when the concentrations of NH 3 (aq), NH 4 + (aq),...
-
Use thermodynamic data at 298 K to decide in which direction the reaction is spontaneous when the partial pressures of SO 2 , O 2 , and SO 3 are 1.0 x 10 -4 , 0.20, and 0.10 bar, respectively. 2...
-
Use thermodynamic data at 298 K to decide in which direction the reaction is spontaneous when the partial pressures of H 2 , Cl 2 , and HCl are all 0.5 bar. Cl(g) 2 HCI(g) H(g) + Cl(g)
-
A company had average total assets of $500,000, gross sales of $575,000, and net sales of $550,000. The companys total asset turnover is a. 1.15. b. 1.10. c. 0.91. d. 0.87. e. 1.05.
-
Gundy Corporation produces area rugs. The following per unit cost information is available: direct materials $18, direct labor $9, variable manufacturing overhead $5, fixed manufacturing overhead $6,...
-
Figure Q25.4 shows four different loops in a magnetic field. The numbers indicate the lengths of the sides and the strength of the field. Rank in order the magnetic fluxes \(\Phi_{1}\) through...
-
Describe the following six ways of improving the effectiveness of studying: (1) elaborate; (2) generate and test; (3) organize; (4) take breaks; (5) match learning and testing conditions; (6) avoid...
-
The following information, taken from the books of Nicholas Company, represents the operations for the month of January: The job cost system is used, and the February cost sheet for Job M45 shows the...
-
work Saved Pearl Products Limited of Shenzhen, China, manufactures and distributes toys throughout South East Asia. Three cubic centimeters (cc) of solvent H300 are required to manufacture each unit...
-
For the reaction 2 NO(g) + O 2 (g) 2 NO 2 (g) all but one of the following equations is correct. Which is incorrect, and why? (a) K = K p ; (b) r S = ( r H - r G)/T; (c) K = e - r G/RT ; (d) r G...
-
In the synthesis of gaseous methanol from carbon monoxide gas and hydrogen gas, the following equilibrium concentrations were determined at 483 K: [CO(g)] = 0.0911 M, [H 2 (g)] = 0.0822 M, and 3[CH 3...
-
Assuming the same facts as in Question 1, what would be the advantages and disadvantages of a joint venture with a major foreign company abroad compared to the alternatives discussed in Question 1?
-
3. (20 points) A researcher is interested in whether the phonics method of teaching reading is more or less effective than the sight method, depending on what grade the child is in. Twenty children...
-
Let A and B be the matrices given below: -5 9 -7 A= 8 -1 -3 B=9 6 -1 8 -1 -7. 0 Perform the following matrix operations and enter the entries below: -4A = A-4B = 5A-3B=
-
The product business can be isolated into four principal classes: programming administrations, framework administrations, open source and SaaS. The accompanying depicts the classifications of...
-
In Exercises 21-24, use these results from the "1-Panel-THC" test for marijuana use, which is provided by the company Drug Test Success: Among 143 subjects with positive test results, there are 24...
-
Please attached excel solution with formulars. An electronics manufacturer wants to expand its market in Europe. The demand in Europe is forecasted as: England France Spain Germany Italy Sweden 9 16...
-
The total power measured in a three-phase system feeding a balanced wye-connected load is 12 kW at a power factor of 0.6 leading. If the line voltage is 208 V, calculate the line current IL and the...
-
If the jobs displayed in Table 18.24 are processed using the earliestdue-date rule, what would be the lateness of job C? TABLE 18.24 Processing Times and Due Dates for Five Jobs Job C D E...
-
Multinational transfer pricing, global tax minimization. Industrial Diamonds, Inc., based in Los Angeles, has two divisions: South African Mining Division, which mines a rich diamond vein in South...
-
International transfer pricing, taxes, goal congruence. Argone Division of Gemini Corporation is located in the United States. Its effective income tax rate is 20%. Another division of Gemini,...
-
Transfer pricing, goal congruence. The Orsilo Corporation makes end sells 10,000 multisystem music players each year Its Assembly Division purchases components from other divisions of Orsilo or from...
-
1. It costs $250 more per month to lease a hybrid (which gives 70mpg) than a non-hybrid (which gives 25mpg). The lease is for 3 years. How much must gas cost per gallon if you choose the hybrid...
-
The stock of Lead Zeppelin, a metal manufacturer, currently sells for $80 and has an annual standard deviation of 35 percent. The risk-free rate is 4 percent. What is the value of a put option with a...
-
PLEASE ANSWER (a) through (i) D E E F B 4.5 4 3.5 6 4 00 0 5 4 O O 6 O 00 6 9 240 360 270 2160 180 1440 210 1680 1920 2880 150 0 90 0 0 0 0 120 600 0 150 90 90 120 120 0 750 450 450 480 600 930 1800...

Study smarter with the SolutionInn App


