For the titration of 25.00 mL 0.100 M NH 3 with 0.100 M HCl, calculate the pOH
Question:
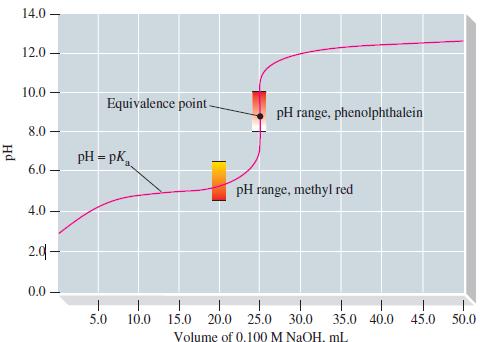
For the titration of 25.00 mL 0.100 M NH3 with 0.100 M HCl, calculate the pOH at a few representative points in the titration, sketch the titration curve of pOH versus volume of titrant, and show that it has exactly the same form as Figure 17-10. Then, using this curve and the simplest method possible, sketch the titration curve of pH versus volume of titrant.
Figure 17-10
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:




