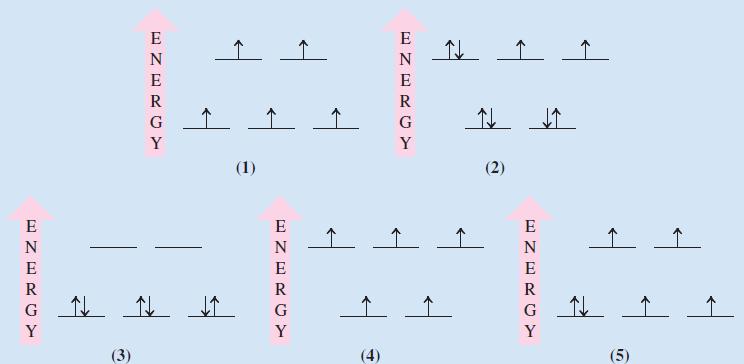
From the following crystal field splitting diagrams identify: (i) A tetrahedral Mn 2+ complex; (ii) A strong-field
Question:
From the following crystal field splitting diagrams identify:
(i) A tetrahedral Mn2+ complex;
(ii) A strong-field octahedral complex of Co3+;
(iii) A weak-field octahedral complex of Fe2+;
(iv) A tetrahedral Ni2+ complex;
(v) A high-spin octahedral complex of Fe3+.
Transcribed Image Text:
E N E R G Y N (3) EN E R G Y N (1) EN RGY ↑ (4) E R G Y N (2) E N E R G Y (5)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
Based on the crystal field splitting diagrams providedthe following can be identified Crystal field ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The [Ni(CN)4]2- ion, which has a square-planar geometry, is diamagnetic, whereas the [NiCl4]2- ion, which has a tetrahedral geometry, is paramagnetic. Show the crystal field splitting diagrams for...
-
Cebo Zikode started Formula Trucks Inc in 1999 and is famous in the racing business and has worked with many different suppliers. Formula Trucks Inc. manufactures high quality trucks, purchases and...
-
For the following crystal field diagrams, label each as low spin, high spin, or cannot tell. Explain your answers. a. b. c.
-
If the molecular weight of air is 28.9, what is the density of air at atmospheric pressure and a temperature of 328.2 K? 1 atm = 1.013 x 10 5 N/m, the mass of a proton is 1.67262 x 10 -27 kg,...
-
Big Company purchased Little Company for $290,000. At the time of purchase, the fair value of Little Companys assets and liabilities was as follows: Inventory . . . . . . . . . . . . . . . . . . . ....
-
Forten Company, a merchandiser, recently completed its calendar-year 2016 operations. For the year, (1) All sales are credit sales, (2) All credits to Accounts Receivable reflect cash receipts from...
-
From calls made with randomly generated telephone numbers, 1012 respondents are asked if they rent or own their residences.
-
Miller Manufacturing makes several different products for the mountain biking enthusiast. In an extremely competitive market, Miller has assumed a strong position by stressing cost control. Several...
-
Need urgent Exercise 10-6 On July 1, 2017, Granville Ltd borrowed $15,400 bry signing a two-year,4% note payable. The note is payable in two annual blended pridalandheres instalmer June 30. Adjusting...
-
Draw a plausible structure to represent: (a) [FeBr(ox) 2 ] 2 (b) [CoBr 4 (NH 3 ) 2 ] (c) [Cu(EDTA)] 2 (d) [CrBr 2 (H 2 O) 4 ] + (e) [PtBr 6 ] 2 .
-
(A) The color of [Co(H 2 O) 6 ] 2+ is pink, whereas that of tetrahedral [CoCl 4 ] 2 is blue. Explain this difference in color. (B) One of the following solids is yellow, and the other is green: Fe(NO...
-
In Exercises locate any relative extrema and points of inflection. Use a graphing utility to confirm your results. y X In x
-
Listed below are the lead concentrations (in ug/g) measured in different Ayurveda medicines. Ayurveda is a traditional medical system commonly used in India. The lead concentrations listed here are...
-
The assignment states to use a movie and talk about 2 scenes where physics ideas are used. The rubric is shown and 6 big ideas that can be talked about are also attached. Background Information...
-
A series of computer and backup system failures caused the loss of most of the company records at Stotter, Incorporated. Information technology consultants for the company could recover only a few...
-
Future value of an annuity Using the values below, answer the questions that follow. (Click on the icon here in order to copy the contents of the data table below into a spreadsheet.) Deposit period...
-
Mercury, Incorporated, produces cell phones at its plant in Texas. In recent years, the company's market share has been eroded by stiff competition from overseas. Price and product quality are the...
-
Complete the following table dealing with affine planes. Number of Number of Field Number of Points Number of Lines Points on a Line Lines on a Point 25 GF32) 56 17 31
-
Bobbie Singh provides writing services for small businesses. He blogs for companies that need professionally written content. His business records at November 15, 2023, are shown below: During the...
-
The unadjusted trial balance for Sierra Corp. is shown in Illustration 4-4 (page 168). In lieu of the adjusting entries shown in the text at October 31, assume the following adjustment data. 1....
-
The income statement of Kaleta Co. for the month of July shows net income of $1,500 based on Service Revenue $5,500; Salaries and Wages Expense $2,100; Supplies Expense $900, and Utilities Expense...
-
This is a partial adjusted trial balance of Fenske Company. InstructionsAnswer these questions, assuming the year begins January 1.(a) If the amount in Supplies Expense is the January 31 adjusting...
-
Construction of consumer price index number for the given goods and services. Item Weight in % Base period price Current period price Food 35 150 145 Fuel 10 25 23 Cloth 20 75 65 Rent 15 30 30 Misc....
-
Gammaro Corporation has found that 80% of its sales in any given month are credit sales, while the remainder are cash sales of the credit sales, Gammaro Corporation has experienced the following...
-
Swifty Company estimates that 2022 sales will be $43,200 in quarter 1,$51,840 in quarter 2 , and $62,640 in quarter 3 , Cost of goods sold is 50% of sales. Management desires to have ending...

Study smarter with the SolutionInn App


