In biochemical reactions* the phosphorylation of amino acids is an important step. Consider the following two reactions
Question:
In biochemical reactions* the phosphorylation of amino acids is an important step. Consider the following two reactions and determine whether the phosphorylation of arginine with ATP is spontaneous.
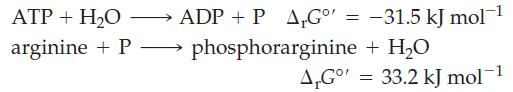
Transcribed Image Text:
ADP + P A₁Gº' = -31.5 kJ mol-¹ phosphorarginine + H₂O ATP + H₂O arginine + P→→→→→→ A,Go' = 33.2 kJ mol-1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
To determine whether the phosphorylation of arginine with ATP is spontaneous we need to compare the ...View the full answer

Answered By

Shaira grace
I have experience of more than ten years in handing academic tasks and assisting students to handle academic challenges. My level of education and expertise allows me communicate eloquently with clients and therefore understanding their nature and solving it successfully.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The racemization of amino acids is an important reaction in a number of bacteria. This is a pyridoxal-phosphate catalyzed reaction. Outline a curved-arrow mechanism for this reaction showing clearly...
-
Behind terrorism is a complex endeavor, shaped by various factors and objectives unique to each terrorist group. In Max Abrahms article, "What Terrorists Really Want: Terrorist Motives and...
-
Proteins are made up of individual molecular units of unique structure known as amino acids. The order or sequence of amino acids is an important factor in determining protein structure and function....
-
For each of the following tests, identify two different samples of people who would have the expertise to serve as subject matter experts (SMEs) for providing judgments regarding the content validity...
-
Urbina Inc. is preparing its annual budgets for the year ending December 31, 2014. Accounting assistants furnish the following data. An accounting assistant has prepared the detailed manufacturing...
-
You are bowling with a 10-pound ball that you throw at 10 mph, and youve decided that you need to hit the pins with a ball that has more momentum. What are two possible changes you could make?
-
What steps would one take in identifying appropriate knowledge management solutions? Briefly describe them.
-
Portland Pale Ale, Inc., makes a variety of specialty beers at its main brewery in Oregon. Production of beer occurs in three main stages: mashing, boiling, and fermenting. Consider the fermenting...
-
Instructions Prepare journal entries that should be made in 2022 and 2023 to record the transactions related to the sales of candy bars, purchase inventory of premium, premium expense and premium...
-
Following are some standard Gibbs energies of formation, f G, at 1000 K: NiO(s), -115 kJ mol -1 ; MnO(s), -280 kJ mol -1 ; TiO 2 (s), -630 kJ mol -1 . The standard Gibbs energy of formation of CO(g)...
-
For the reaction N 2 O 4 (g) 2 NO 2 (g), r H = +57.2 kJ mol -1 and K = 0.113 at 298 K. (a) What is K at 0 C? (b) At what temperature will K = 1.00?
-
Go to www.ponemon.org/local/upload/file/2013_US _Cost_of_Cyber_Crime_Study_FINAL6%20.pdfanddownload the 2013 report (or a more recent report if one is available). a. Summarize the survey with regard...
-
Instructions FNCE 625 - Investment Analysis and Management Group Project - Case Study Guideline Introduction: In this group assignment, each team will collaboratively make a comprehensive report and...
-
21) The EOQ model is solved using calculus but the key intuition is that relevant total costs are minimized when relevant ordering costs equal relevant carrying costs. 22) Safety stock is used as a...
-
In the long-term, what do you recommend as overall policy in order to reduce or avoid the kinds of PPE shortages that occurred during the different waves of the COVID virus? In simple terms, how...
-
Which topics do you see as being most relevant to your current job or the job you will seek to obtain once you have earned your degree? How so ? In which ways has this course Commercial Law changed...
-
Directions Answer the following reflective questions: There do exist examples of business organizations following principles of behavior that are not entirely self-serving, but rather, are pursuing...
-
For the circuit shown in Fig. 13.110, find the value of the average power absorbed by the 8- resistor. 4862 4 sin (30) v (-) 862
-
An 8.0 kg crate is pulled 5.0 m up a 30 incline by a rope angled 18 above the incline. The tension in the rope is 120 N, and the crates coefficient of kinetic friction on the incline is 0.25. a. How...
-
Production-Volume Variance Analysis and Sales Volume Variance. Dawn Floral Creations, Inc., makes jewelry in the shape off flowers. Each piece is hand-made and takes an average of 1.5 hours to...
-
Comprehensive review of Chapters working backward from given variances. The Mancusco Company uses a flexible budget and standard costs to aid planning and control of its machining manufacturing...
-
The Beal Manufacturing Companys costing system has two direct-cost categories: direct materials and direct manufacturing labor. Manufacturing overhead (both variable and fixed) is allocated to...
-
Kindly Provide brief answers to the following: a) Why do we add floatation costs in the calculations of individual components costs? b) List and briefly explain the qualitative and quantitative...
-
Which of the following statements is correct. On average, the most efficient strategy to create value for existing shareholders is: To buy back shares To offer new products in a growing market To...
-
Which investment should I choose? Bond X: AA Corporate bond, Par=$1,000, Coupon rate=5% (semiannual coupons), 5 years to maturity Bond Y: AA Corporate bond, Par=$5,000, Coupon rate=5.5% (semiannual...

Study smarter with the SolutionInn App


