In the diagram below, the sketch on the far left represents the [H 3 O + ]
Question:
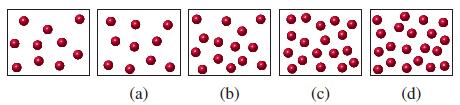
In the diagram below, the sketch on the far left represents the [H3O+] present in an acetic acid solution of molarity c. If the molarity of the solution is doubled, which of the sketches below best represents the resulting solution?
Transcribed Image Text:
(a) (b) (c) (d)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
If the molarity of the acetic acid solution is doubled the concentration ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Able and Body are unrelated individuals. In September of the present year, they decide to form I Can Too Corporation. Able contributes a building and land with a basis of $35,000 and and a fair...
-
In the diagram below, the sketch on the far left represents the [OH - ] present in an ammonia solution of molarity c. If the solution is diluted to half its original molarity, which of the sketches...
-
What must be the molarity of an acetic acid solution if it has the same percent ionization as 0.100 M CH 3 CH 2 CO 2 H (propionic acid, K a = 1.3 x 10 -5 )?
-
Rewe Company's income statement contained the condensed information below. Rewe's balance sheets contained the following comparative data at December 31. Accounts payable pertain to operating...
-
What is the conversion feature? What is a conversion ratio? How do convertibles and other contingent securities affect EPS? Briefly describe the motives for convertible financing.
-
A salesperson makes four calls per day. A sample of 100 days gives the following frequencies of sales volumes. Records show sales are made to 30% of all sales calls. Assuming independent sales calls,...
-
6. You are analyzing a distressed bond with one year to maturity. The bond has a face value of $100 and pays a coupon rate of 5 percent per year, with annual coupons. The bond is currently trading at...
-
Why did Toyota wait so long to publically acknowledge and replace the faulty accelerator pedals? Toyota is an extremely successful automaker that has built a reputation for quality by fostering a...
-
Which of the following about rainbow options is not correct? It is difficult to value rainbow options because the number of nodes in the binomial tree grows exponentially with the number of risky...
-
One handbook lists a value of 9.5 for pK b of quinoline, C 9 H 7 N, a weak base used as a preservative for anatomical specimens and to make dyes. Another handbook lists the solubility of quinoline in...
-
A 275 mL sample of vapor in equilibrium with propan-1-amine at 25.0 C is removed and dissolved in 0.500 L H 2 O. For propan-1-amine, pK b = 3.43 and v.p. = 316 Torr. (a) What should be the pH of the...
-
The cooling water from the condenser of a power plant enters a wet cooling tower at 105oF at a rate of 90 lbm/s. The water is cooled to 85oF in the cooling tower by air which enters the tower at 1...
-
Consider the following account balances (in thousands) for the Shaker Corporation In the Dec 31.2021 Cash $200,000 and Capital $2,000,000 and Retained earnings $1,500,000 The balances of raw...
-
Unless otherwise stated, assume gravitational acceleration g = 9.81 m/s and the density of water to be 1000 kg/m. Unless otherwise stated, give all numerical answers to 3 significant figures, such as...
-
The purpose of this installment is to classify stock, bond, and mutual fund investments, explore tools for their evaluation and select these securities based on your investment philosophy and goals....
-
Jackson County Senior Services is a nonprofit organization devoted to providing essential services to seniors who live in their own homes within the Jackson County area. Three services are provided...
-
Caldwell (2003) explores differences between the roles of leaders and managers. "Leaders...envision, initiate, or sponsor strategic change of a far-reaching or transformational nature. In contrast,...
-
Emron Inc. is considering these two alternatives to finance its construction of a new $2 million plant: 1. Issuance of 200,000 shares of common stock at the market price of $10 per share. 2. Issuance...
-
Illini Company, Inc. Balance Sheet as of 12/31/20X0 Assets Current Assets: Cash $1,500,000 Accounts receivable, net 18,000 Inventory 50,000 Total current assets 1,568,000 Equipment 90,000 Goodwill...
-
The following data were taken from the balance sheet of Bock Suppliers Company: (a) Determine for each year(1) The working capital,(2) The current ratio, and(3) The quick ratio. Round ratios to one...
-
PepsiCo, Inc., the parent company of Frito-Lay snack foods and Pepsi beverages, had the following current assets and current liabilities at the end of two recent years: (a) Determine the(1) Current...
-
The bond indenture for the 10-year, 10% debenture bonds dated January 2, 2009, required working capital of $142,000 a current ratio of 1.7, and a quick ratio of 1.2 at the end of each calendar year...
-
Fig 1. Rolling a 4 on a D4 A four sided die (D4), shaped like a pyramid (or tetrahedron), has 4 flat surfaces opposite four corner points. A number (1, 2, 3, or 4) appears close to the edge of each...
-
I just need help with question #4 please! Thank you! Windsor Manufacturing uses MRP to schedule its production. Below is the Bill of Material (BOM) for Product A. The quantity needed of the part...
-
(25) Suppose that we have an economy consisting of two farmers, Cornelius and Wheaton, who unsurprisingly farm corn c and wheat w, respectively. Assume that both farmers produce their crop of choice...

Study smarter with the SolutionInn App


