In the qualitative cation analysis procedure, Bi 3+ is detected by the appearance of a white precipitate
Question:
In the qualitative cation analysis procedure, Bi3+ is detected by the appearance of a white precipitate of bismuthyl hydroxide, BiOOH(s):
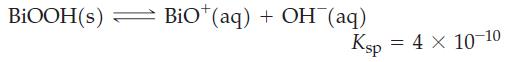
Calculate the pH of a saturated aqueous solution of BiOOH.
Transcribed Image Text:
BIOOH(s) BiO (aq) + OH(aq) Ksp = 4 X 10-10
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To calculate the pH of a saturated solution of bismuthyl hydroxide BIOOH we can use the solubility p...View the full answer

Answered By

Danish Sohail
My objective is to become most reliable expert for clients. For last 10 years I have been associated with the field of accounting and finance. My aim is to strive for best results and pay particular attention to client needs. I am always enthusiastic to help clients for issues and concerns related to business studies. I can work on analysis of the financial statements, calculate different ratios and analysis of ratios. I can critically evaluate stock prices based on the financial analysis and valuation for companies using financial statements of the business entity being valued with use of excel tools. I have expertise to provide effective and reliable help for projects in corporate finance, equity investments, financial accounting, cost accounting, financial planning, business plans, marketing plans, performance measurement, budgeting, economic research, risk assessment, risk management, derivatives, fixed income investments, taxation, auditing, and financial performance analysis.
4.80+
78+ Reviews
112+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
The composition of rewards applied by an organization and its association with performance management system can shape organizational culture, Do you agree or disagree and why?
-
1.) Let G be an n-partite simple graph, the partite sets of which are {ui, vi}, {u2, v2}, {un, Un}. Assume that the sequence of degrees d(v), d(u2), d(v2),..., d(un-1), d(vn-1), d(un), d(vn) is some...
-
FinCorp Inc. is exploring different portfolio allocations between two stocks. Complete the following table. Case1 Case 2 Case 3 Case 4 Case 5 $ invested in stock 1 $ invested in stock 2 Total $...
-
Distinguish between taxable temporary differences and deductible temporary differences, and give at least two examples of each type.
-
A $100 000 bond is redeemable at par in 14 years, 10 months. If interest on the bond is 7.5% payable semiannually, what is the purchase price to yield 8% com- pounded semiannually? (a) What is the...
-
Using the WVS dataset, create a grouped frequency distribution of the variable % OF COUNTRY THAT SAYS THEY GET THEIR NEWS THROUGH THEIR MOBILE PHONES. Use the following categories: 0 9, 1019, 2029,...
-
Super Clean, Inc. produces and sells stain remover. Information about the budget for the year 2012 is as follows: 1. The company expects to sell 50,000 bottles of stain remover in the first quarter,...
-
Exercise 22-6 Departmental expense allocation spreadsheet LO P2 Marathon Running Shop has two service departments (advertising and administrative) and two operating departments (shoes and clothing)....
-
A solution is saturated with magnesium palmitate [Mg(C 16 H 31 O 2 ) 2 , a component of bathtub ring] at 50 C. How many milligrams of magnesium palmitate will precipitate from 965 mL of this solution...
-
Fluoridated drinking water contains about 1 part per million (ppm) of F. Is CaF 2 sufficiently soluble in water to be used as the source of fluoride ion for the fluoridation of drinking water?...
-
(A) Equilibrium is established in a 3.00 L flask at 1405 K for the reaction 2 H 2 S(g) 2 H 2 (g) + S 2 (g). At equilibrium, there is 0.11 mol S 2 (g), 0.22 mol H 2 (g), and 2.78 mol H 2 S(g). What...
-
1. Mainland purchased a machine for $85,000 on 1 January 20x7 and assigned it a useful life for 10 years. On 31 March 20x9 it was revalued to $93,000 with no change in useful life. Complete the table...
-
Find the equation of the regression line and identify a characteristic of the data that is ignored by the regression line X 10 8 13 9 11 14 6 4 12 7 5 Y 7.46 6.77 12.74 7.11 7.81 8.84 6.08 5.39 8.15...
-
For each of the following independent cases, fill in the missing amounts in the table: (Indicate the effect of each variance by selecting "F" for favorable, "U" for unfavorable.) Case Direct Labor...
-
All views expressed in this paper are those of the authors and do not necessarily represent the views of the Hellenic Observatory or the LSE George Alogoskoufis Greeces Sovereign Debt Crisis:...
-
Current Attempt in Progress Nash Company is constructing a building. Construction began on February 1 and was completed on December 31. Expenditures were $1,812,000 on March 1, $1,212,000 on June 1,...
-
Inspect Table 8.6 and identify tool materials that would not be particularly suitable for interrupted cutting operations, such as milling. Explain your choices.
-
Horse serum containing specific antibody to snake venom has been a successful approach to treating snakebite in humans. How do you think this anti-venom could be generated? What are some advantages...
-
(a) Jenny Kent asks your help in understanding the term activity index. Explain the meaning and importance of this term for Jenny. (b) State the two ways that variable costs may be defined.
-
A. J. Hernandez claims that the relevant range concept is important only for variable costs. (a) Explain the relevant range concept. (b) Do you agree with A. J.s claim? Explain.
-
The relevant range is indispensable in cost behavior analysis. Is this true? Why or why not?
-
Maddox Resources has credit sales of $ 1 8 0 , 0 0 0 yearly with credit terms of net 3 0 days, which is also the average collection period. Maddox does not offer a discount for early payment, so its...
-
Selk Steel Co., which began operations on January 4, 2017, had the following subsequent transactions and events in its long-term investments. 2017 Jan. 5 Selk purchased 50,000 shares (25% of total)...
-
Equipment with a book value of $84,000 and an original cost of $166,000 was sold at a loss of $36,000. Paid $100,000 cash for a new truck. Sold land costing $330,000 for $415,000 cash, yielding a...

Study smarter with the SolutionInn App


