One sketch below represents an initial nonequilibrium mixture in the reversible reaction Which of the other three
Question:
One sketch below represents an initial nonequilibrium mixture in the reversible reaction
![]()
Which of the other three sketches best represents an equilibrium mixture? Explain.
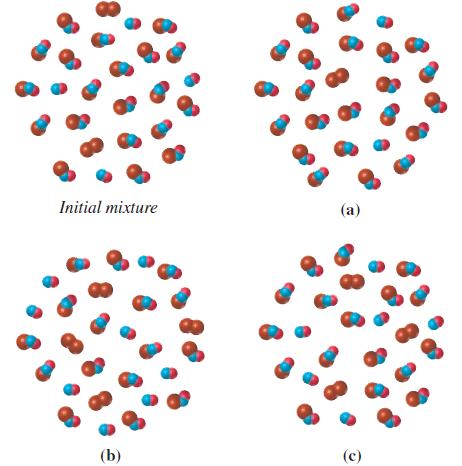
Transcribed Image Text:
2 NO(g) + Br₂(g) = 2 NOBr(g) Kc = 3.0
Step by Step Answer:

This question has not been answered yet.
You can Ask your question!
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Have regulatory changes occurring since 2000 had a significant positive or negative impact on the manner in which board of directors operate? Explain
-
One sketch below represents an initial nonequilibrium mixture in the reversible reaction Which of the other three sketches best represents an equilibrium mixture? Explain. Kc = 4.0 (8) (8) + (8)
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
Assume your company shows the market values of equity and debt at the level of $175373 and $224626, respectively. The rate of return on assets is 33 percent and its volatility is 45 percent. The...
-
Max Small has outstanding school loans that require a monthly payment of $1,000. He needs to purchase a new car for work and estimates that this will add $350 per month to his existing monthly...
-
Why dont the state courts all just adopt the same type of procedure for filing appeals that is used by the U.S. Supreme Court?
-
3. Define maturity mismatch. Why is maturity mismatch important for understanding a banks risk and analyzing its performance?
-
Shown on the next page are comparative income statements for McDonald's for 2005, 2006, and 2007. 1. There are two kinds of McDonald's restaurants'restaurants that McDonald's itself owns and...
-
Total cost of one unit is equal to R500. If the mark-up on cost is 30%, calculate the price of one unit. Show the relevant equation
-
Lead metal is added to 0.100 M Cr 3+ (aq). What are [Pb 2+ ], [Cr 2+ ], and [Cr 3+ ] when equilibrium is established in the reaction? 3+ Pb(s) + 2 Cr+ (aq) = Pb+ (aq) + 2 Cr+ (aq) K 3.2 x 10-10 =
-
Cadmium metal is added to 0.350 L of an aqueous solution in which [Cr 3+ ] = 1.00 M. What are the concentrations of the different ionic species at equilibrium? What is the minimum mass of cadmium...
-
In the layout of a source document: a. To prevent users from being confused, keying instructions should not appear on the form b. Instructions should not be combined with questions c. Fields should...
-
Company, a soft-drink vendor, has created a table of costs for three stocking decisions for three different states of nature: Alternatives States of Nature Low Demand Medium Demand High Demand Large...
-
utilizes a project scheduling software application to develop a project schedule for a construction project.
-
Kimi is a server or restaurant and relies on tips from a customers to make a living. She doesn't really enjoy her job and frequently think about quitting because she is constantly having 2% a happy...
-
What represents revenues (Inflow) and expenses (Outflow) for a healthcare organization? Your paper should include information on sources of healthcare revenue (governmental and private payers), how...
-
case study analysis should be a thoughtful write up including: 1.a brief case analysis 2.key questions and answers from the case from your unique perspective 3.a summary and/or recommendations...
-
The financial statements of Amazon.com, Inc. are presented in Appendix D. Financial statements of Wal-Mart Stores, Inc. are presented in Appendix E. Instructions (a) Based on the information...
-
Given the table below, about how much force does the rocket engine exert on the 4.0 kg payload? Distance traveled with rocket engine firing (m) Payload final velocity (m/s) 500 320 490 310 1020 450...
-
McLean Company produced 2,500 units of product that required two standard hours per unit. The standard fixed overhead cost per unit is $1.30 per hour at 4,600 hours, which is 100% of normal capacity....
-
Norris Company produced 500 units that require six standard pounds per unit at $1.25 standard price per pound. The company actually used 2,900 pounds in production. Journalize the entry to record the...
-
McLean Company produced 2,500 units that require three standard gallons per unit at $18.50 standard price per gallon. The company actually used 8,000 gallons in production. Journalize the entry to...
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...

Study smarter with the SolutionInn App


