Refer to the discussion of the Leclanch cell. (a) Combine the several equations written for the operation
Question:
Refer to the discussion of the Leclanché cell.
(a) Combine the several equations written for the operation of the Leclanché cell into a single overall equation.
(b) Given that the voltage of the Leclanché cell is 1.55 V, estimate the electrode potentials, E, for each of the half cell reactions. Why are your values only estimates?
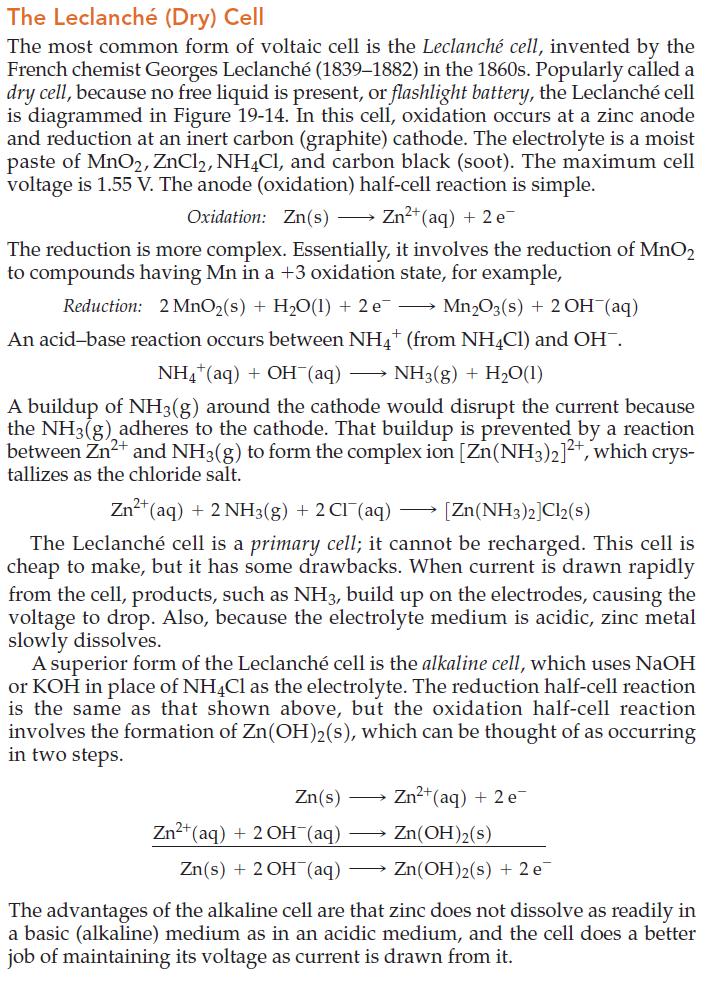
Figure 19.14
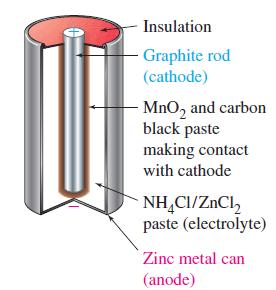
Transcribed Image Text:
The Leclanché (Dry) Cell The most common form of voltaic cell is the Leclanché cell, invented by the French chemist Georges Leclanché (1839-1882) in the 1860s. Popularly called a dry cell, because no free liquid is present, or flashlight battery, the Leclanché cell is diagrammed in Figure 19-14. In this cell, oxidation occurs at a zinc anode and reduction at an inert carbon (graphite) cathode. The electrolyte is a moist paste of MnO2, ZnCl2, NH4Cl, and carbon black (soot). The maximum cell voltage is 1.55 V. The anode (oxidation) half-cell reaction is simple. Oxidation: Zn(s) →→→ Zn²+ (aq) + 2 e The reduction is more complex. Essentially, it involves the reduction of MnO2 to compounds having Mn in a +3 oxidation state, for example, Reduction: 2 MnO₂ (s) + H₂O(1) + 2 e¯ Mn₂O3(s) + 2OH(aq) An acid-base reaction occurs between NH4+ (from NH4Cl) and OH¯. NH4+ (aq) + OH(aq) NH3(g) + H₂O(1) A buildup of NH3(g) around the cathode would disrupt the current because the NH3(g) adheres to the cathode. That buildup is prevented by a reaction between Zn²+ and NH3(g) to form the complex ion [Zn(NH3)2]²+, which crys- tallizes as the chloride salt. → Zn²+ (aq) + 2NH3(g) + 2Cl(aq) [Zn(NH3)2]Cl2(s) The Leclanché cell is a primary cell; it cannot be recharged. This cell is cheap to make, but it has some drawbacks. When current is drawn rapidly from the cell, products, such as NH3, build up on the electrodes, causing the voltage to drop. Also, because the electrolyte medium is acidic, zinc metal slowly dissolves. A superior form of the Leclanché cell is the alkaline cell, which uses NaOH or KOH in place of NH4Cl as the electrolyte. The reduction half-cell reaction is the same as that shown above, but the oxidation half-cell reaction involves the formation of Zn(OH)2(s), which can be thought of as occurring in two steps. Zn(s) Zn²+ (aq) + 2 e Zn²+ (aq) + 2OH(aq) Zn(OH)2(s) Zn(s) + 2OH(aq) → Zn(OH)2(s) + 2 e The advantages of the alkaline cell are that zinc does not dissolve as readily in a basic (alkaline) medium as in an acidic medium, and the cell does a better job of maintaining its voltage as current is drawn from it.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 87% (8 reviews)

Answered By

Brown Arianne
Detail-oriented professional tutor with a solid 10 years of experience instilling confidence in high school and college students. Dedicated to empowering all students with constructive feedback and practical test-taking strategies. Effective educator and team player whether working in a school, university, or private provider setting. Active listener committed to helping students overcome academic challenges to reach personal goals.
4.60+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Refer to the discussion of the automobiles in the section on "TradingOff Conflicting Objectives: The Basics." We discussed switching first from the Standard to the Norushi, and then from the Norushi...
-
Refer to the discussion of beta values and market models in Problem 13.49 on page 553. The S&P 500 Index tracks the overall movement of the stock market by considering the stock prices of 500 large...
-
Refer to the discussion of Benfords law in Exercise 7.25. While this may seem like a curious oddity, researchers have developed some important applications for these proportions. One involves...
-
You are developing an industrial building with a gross building area of 150,000 sf. The building efficiency ratio is 75%. The market gross rent is $25 psf. The vacancy rate is 5%; the cap rate is 5%;...
-
Discuss the problems that might be encountered in having an expatriate sales manager supervising foreign salespeople.
-
You can access and download the model as well as detailed instructions for further analysis within the Chens End of Chapter Questions.zip file on the book web site. You will be asked to create and...
-
Describe the two types of error possible in a hypothesis test decision.
-
HealthSouth Corporation claims to be . . . the nations largest owner and operator of inpatient rehabilitation hospitals in terms of revenues, number of hospitals, and patients treated and discharged....
-
The glasses will sell for $ 1 , 5 0 0 per pair and have a variable cost of $ 5 0 0 per unit. The company has spent $ 1 3 5 , 0 0 0 for a marketing study that estimates the company will sell 1 0 4 , 0...
-
For the voltaic cell, (a) what isE cell initially? (b) As the cell operates, willE cell increase, decrease, or remain constant with time? Explain. (c) What will beE cell when [Ag + ] has increased to...
-
For the voltaic cell, (a) What is E cell initially? (b) If the cell is allowed to operate spontaneously, willE cell increase, decrease, or remain constant with time? Explain. (c) What will beE cell...
-
Granger Products recorded the following transactions for the just completed month. The company had no beginning inventories. a. $75,000 in raw materials were purchased for cash. b. $73,000 in raw...
-
Recognition is a very important element of volunteer management. Do you know someone who has done amazing volunteer work for a good cause? Wouldn't it be nice to thank them with an award! Take a look...
-
What do Financial Planners do? Would you consider hiring a Financial Planner? How important are ethics when working with a financial planning professional? Explain the concept of return on...
-
Explain the specific perceptual errors you made of EACH of your teammates during the class exercise
-
Identify the company that makes the product a. Are they a large company or a small company? b. Are they a chain or a major corporation? c. Have they been around for decades or are they a new company?...
-
The Red Inn at Pismo is a 150-room hotel that caters mainly to business clients during the week and to tourists during the weekends and in the summer. Below is a table summarizing the average daily...
-
Answer these questions about 2 companies. 1. Peru, Inc., began the year with total liabilities of $140,000 and total stockholders' equity of $300,000. During the year, total assets increased by 20%....
-
TRUE-FALSE QUESTIONS 1. In terms of preliminary analytical procedures, assume that the company has introduced a new product with a low price point and significant customer demand. The auditor would...
-
Based on the data presented in Exercise 25-21, assume that Ohio Glass wanted to price all products so that they produced the same profit potential as the highest profit product. Thus, determine the...
-
Cardio Care Inc. manufactures stationary bicycles and rowing machines. The products are produced in the Fabrication and Assembly production departments. In addition to production activities, several...
-
Titan Industries manufactures two types of electrical power units, custom and standard, which involve four overhead activities?production setup, procurement, quality control, and materials...
-
A firm purchased a new piece of equipment with an estimated useful life of eight years. The cost of the equipment was $65,000. The salvage value was estimated to be $10,000 at the end of year 8....
-
5. Which of the following is the cheapest for a borrower? a. 6.7% annual money market basis b. 6.7% semi-annual money market basis c. 6.7% annual bond basis d. 6.7% semi-annual bond basis.
-
Waterloo Industries pays 30 percent corporate income taxes, and its after-tax MARR is 24 percent. A project has a before-tax IRR of 26 percent. Should the project be approved? What would your...

Study smarter with the SolutionInn App


