Select the correct answer and explain your reasoning. An electron having n = 3 and me =
Question:
Select the correct answer and explain your reasoning.
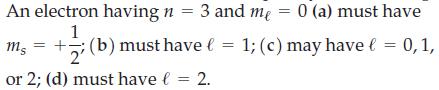
Transcribed Image Text:
An electron having n = 3 and me = 0 (a) must have 1 ms =+; (b) must have l = 1; (c) may have € = 0, 1, 2' or 2; (d) must have l = 2.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The given information specifies that the electron has the following quantum numbers n 3 ml 3 ms ...View the full answer

Answered By

Rohail Amjad
Experienced Finance Guru have a full grip on various sectors, i.e Media, Insurance, Automobile, Rice and other Financial Services.
Have also served in Business Development Department as a Data Anlayst
4.70+
32+ Reviews
83+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Select the correct answer for each of the following questions. 1. On January 1, 20X7, the partners of Casey, Dithers, and Edwards, who share profits and losses in the ratio of 5:3:2, decided to...
-
You want to buy a piece of land for $12,000 cash. The owner would allow you to pay for it in six annual installments of $2300 each, the first one right now. Which method is cheaper for you (paying in...
-
Select the correct answer for each of the following questions. 1. On January 2, 20X3, Kean Company purchased a 30 percent interest in Pod Company for $250,000. Pod reported net income of $100,000 for...
-
Internal Company Assessment (Strategic Analysis Output) on Riise Ev converting Canada post delivery cars to electronic finding out firm strengths weakness liabilities problem constraints...
-
How can the provisions of the SarbanesOxley Act help minimize the likelihood of auditors failing to identify accounting irregularities? Arthur Andersen LLP was founded in Chicago in 1913 by Arthur...
-
A $1000, 6.5% coupon bond has 13 years remaining until maturity. Calculate the bond premium if the required return in the bond market is 5.5% compounded semiannually.
-
In your own words, restate how ERP systems eliminate the problems of departmental information silos. Review Figure 2-3 on page 33. If AllRoad had an ERP system, would that system help or hinder the...
-
On January 1, 2013, Carlos Corporation purchases 90% (18,000 shares) of the outstanding common stock of Dower Company for $504,000. Just prior to Carlos Corporations purchase, Dower Company has the...
-
a) During a home design and interior exhibition, an exhibitor received deposits for branded kitchen hoods to be installed in new houses upon completion of house renovation. The installation dates...
-
1. Within days of the triplets arrival, Jamie Lee and Ross began researching and comparing various agencies for the purchase of a life insurance policy. What characteristics should Ross look for when...
-
The greatest probability of finding the electron in a small-volume element of the 1s orbital of the hydrogen atom is at the nucleus. Yet the most probable distance of the electron from the nucleus is...
-
Describe some of the differences between the orbits of the Bohr atom and the orbitals of the wave mechanical atom. Are there any similarities?
-
What are the major steps of systems implementation?
-
Do we drive technology, or does technology drive us? If technology drives us, what are the risks? The other side of the coin would be that we are able to stay ahead of technological transformations....
-
How do you explain the differences between the two analyses and what are the implications of using the BCG matrix in practice?
-
How do leadership styles, such as transformational leadership, shared leadership, and servant leadership, impact team dynamics, member motivation, and overall team effectiveness ?
-
How do these relevant legal principles apply: Duty of care Duty of obedience Duty of loyalty Shareholder Derivative suit Piercing the corporate veil...
-
what will you do as a hotel manager if a customer complained about bad service they received?
-
Write a single equation (using only x and y) that is equivalent to each pair of parametric equations. a. x = 2t - 3 y = t + 2 b. x = t2 y = t + 1 c. x = 1/2t + 1 y = t - 2 / 3 d. x = t - 3 y = 2(t -...
-
What are the before image (BFIM) and after image (AFIM) of a data item? What is the difference between in-place updating and shadowing, with respect to their handling of BFIM and AFIM?
-
Define organizational environment and organizational technology. In what ways do these concepts overlap?
-
Identify and describe some of the environmental and technological factors that affect your college or university. Give specific examples of how they affect you as a student.
-
How does organization design usually differ for large and small organizations?
-
Both answers please Problem - 7 Akimora Dairy began operations on April 1, 2015, with purchase of 250 miking cows for 18.500.000. It has completed the first month of operations and has the following...
-
need help answering questions 3. What is the deferral adjustment associated with deferred revenue? a. Dr. Deferred Revenue (liability - BS), Cr. Revenue (revenue - IS) 4. What is the deferral...
-
Hosmer Corporation issued $190,000 par value, 6%, 4-year bonds (i.e., there were 190 of $1,000 par value bonds in the issue). Interest is payable semiannually each January 1 and July 1 with the first...

Study smarter with the SolutionInn App


