Silver tarnish is mainly Ag 2 S: A tarnished silver spoon is placed in contact with a
Question:
Silver tarnish is mainly Ag2S:
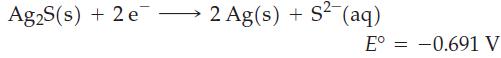
A tarnished silver spoon is placed in contact with a commercially available metallic product in a glass baking dish. Boiling water, to which some NaHCO3 has been added, is poured into the dish, and the product and spoon are completely covered. Within a short time, the removal of tarnish from the spoon begins.
(a) What metal or metals are in the product?
(b) What is the probable reaction that occurs?
(c) What do you suppose is the function of the NaHCO3?
(d) An advertisement for the product appears to make two claims:
(1) No chemicals are involved, and
(2) the product will never need to be replaced. How valid are these claims? Explain.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





