The chief compound in marble is CaCO 3 . Marble has been widely used for statues and
Question:
The chief compound in marble is CaCO3. Marble has been widely used for statues and ornamental work on buildings. However, marble is readily attacked by acids. Determine the solubility of marble (that is, [Ca2+] in a saturated solution) in
(a) Normal rainwater of pH = 5.6;
(b) Acid rainwater of pH = 4.20.
Assume that the overall reaction that occurs is
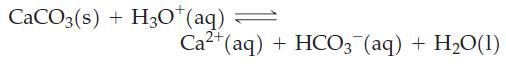
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





