The concept of formal charge helped us to choose the more plausible of the Lewis structures for
Question:
The concept of formal charge helped us to choose the more plausible of the Lewis structures for NO2+ given in expressions (10.14) and (10.15). Can it similarly help us to choose a single Lewis structure as most plausible for CO2H+? Explain.
Eq. 10.14
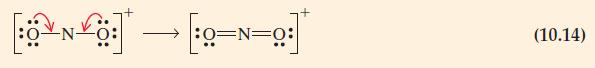
Eq. 10.15

Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





