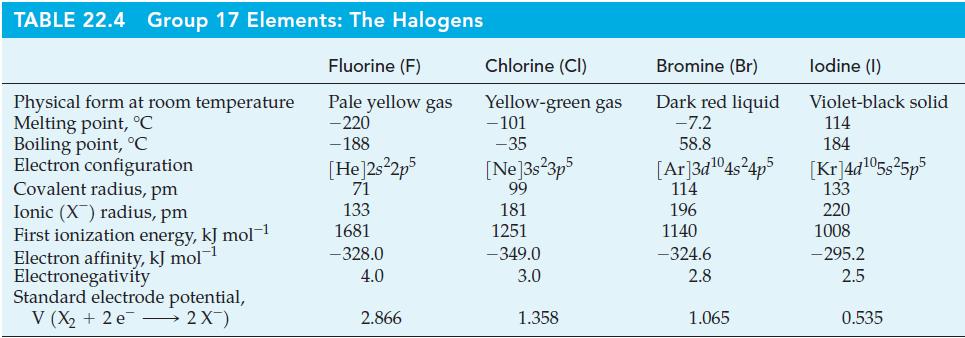
The following properties of astatine have been measured or estimated: (a) Covalent radius; (b) Ionic radius (At
Question:
The following properties of astatine have been measured or estimated:
(a) Covalent radius;
(b) Ionic radius (At–);
(c) First ionization energy;
(d) Electron affinity;
(e) Electronegativity;
(f) Standard reduction potential.
Based on periodic relationships and data in Table 22.4, what values would you expect for these properties?
Table 22.4
Transcribed Image Text:
TABLE 22.4 Group 17 Elements: The Halogens Fluorine (F) Pale yellow gas -220 - 188 Physical form at room temperature Melting point, °C Boiling point, °C Electron configuration Covalent radius, pm Ionic (X) radius, pm First ionization energy, kJ mol-1 Electron affinity, kJ mol-¹ Electronegativity Standard electrode potential, V (X₂ + 2 e 2 X¯) [He]2s²2p5 71 133 1681 -328.0 4.0 2.866 Chlorine (CI) Yellow-green gas -101 -35 [Ne]3s²3p5 99 181 1251 -349.0 3.0 1.358 Bromine (Br) Dark red liquid -7.2 58.8 [Ar]3d¹04s²4p5 114 196 1140 -324.6 2.8 1.065 lodine (1) Violet-black solid 114 184 [Kr]4d¹05s25p5 133 220 1008 -295.2 2.5 0.535
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To make predictions for astatine At we can use the periodic trends and compare it with the halogens ...View the full answer

Answered By

Daniel Kimutai
I am a competent academic expert who delivers excellent writing content from various subjects that pertain to academics. It includes Electronics engineering, History, Economics, Government, Management, IT, Religion, English, Psychology, Sociology, among others. By using Grammarly and Turnitin tools, I make sure that the writing content is original and delivered in time. For seven years, I have worked as a freelance writer, and many scholars have achieved their career dreams through my assistance.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
In the paper, Delayed Metamorphosis of a Tropical Reef Fish (Acanthurus triostegus): A Field Experiment (Marine Ecology Progress Series, Vol. 176, pp. 2538), M. McCormick studied larval duration of...
-
A HartreeFock calculation using the minimal basis set of the 1s, 2s, 2p x , 2p y , and 2p z AOs on each of N and O generated the energy eigenvalues and AO coefficients listed in the following table....
-
3. Using an AD-AS model, can you answer whether a tax that improves people's health could have any potential long-term benefits for the economy.
-
Refer to the data in PE 8-1. Make the journal entry necessary to record salaries expense for the period. Data from PE 8-1 State withholding taxes payable . . . . . . . . . . . . . . . . . . . . . . ....
-
Assume that Temp Force is a constant growth company whose last dividend (Do, which was paid yesterday) was $2.00 and who dividend is expected to grow indefinitely at a 6% rate. (1) What is the firm's...
-
The text describes three characteristics of demand. Name and describe each. LO.1
-
On July 1, 2016, Osceola Company retired a metal stamping machine that it had originally purchased for $1,500,000. At December 31, 2015, the machine had a book value of $125,000 and was being...
-
Please help me explain why the Nash equilibrium is 225,195. Firm XYZ Strategy Low Low 100, 100 Medium 200, 75 High 300, 200 Firm ABC Medium 150, 200 125, 150 100, 225 High 200, 300 225, 195 150, 250
-
Fluorine can be prepared by the reaction of hexafluoro-manganate(IV) ion, MnF 6 2 , with antimony pentafluoride to produce manganese(IV) fluoride and SbF 6 , followed by the disproportionation of...
-
Freshly prepared solutions containing iodide ion are colorless, but over time they usually turn yellow. Describe a plausible chemical reaction (or reactions) to account for this observation.
-
Brady Company has 30,000 shares of $10 par value common stock authorized and 20,000 shares issued and outstanding. On August 13, 2019, Brady purchased 1,000 shares of treasury stock for $12 per...
-
Identify at least two business systems that support the development of effective work relationships Briefly explain how each system supports the development of effective work relationships.
-
Power and Influence Personal Plan - How will you navigate the realms of power and influence? Why is this personal plan important for you? What do you want to achieve? do a table with SMART goals -...
-
A single-stage trickling-filter plant is proposed for treating a dilute wastewater with a BOD concentration of 170 mg/L. The plant is located in a warm climate, and the minimum wastewater temperature...
-
For the first assignment for this course, compose a written document that contains the following: A description and assessment of your past experiences with policy and program planning, either your...
-
What are the key motivators driving consumer purchasing decisions in our industry? How do consumers perceive our brand compared to competitors, and what factors influence brand loyalty?
-
(a) How many distinct terms does the linear congruential generator with a = 5, c = 3, m = 19, and x0 = 10, produce? (b) What is the sequence of pseudorandom members generated?
-
What mass of KBr (in grams) should you use to make 350.0 mL of a 1.30 M KBr solution?
-
The financial statements of The Hershey Company are presented in Appendix B, following the financial statements for Tootsie Roll in Appendix A. Instructions Answer the following questions for each...
-
The website www.cpa2biz.com has an article dated February 4, 2010, by Mary Schaeffer entitled Emerging Issues: Demise of Paper Checks. Instructions Go to the website and do a search on the article...
-
The international accounting firm Ernst and Young performed a global survey. The results of that survey are summarized in a report titled Fraud Risk in Emerging Markets. do an Internet search for 9th...
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer
-
Domino is 4 0 years old and is married out of community of property with the exclusion of the accrual system to Dolly ( 3 5 ) . They have one child, Domonique, who is 1 1 years old. Domino resigned...
-
YOU ARE CREATING AN INVESTMENT POLICY STATEMENT FOR JANE DOE General: 60 years old, 3 grown children that are living on their own and supporting themselves. She is in a very low tax rate so we don't...

Study smarter with the SolutionInn App


