The ionization constants of ortho-phthalic acid are K a1 = 1.1 x 10 -3 and K
Question:
The ionization constants of ortho-phthalic acid are Ka1 = 1.1 x 10-3 and Ka2 = 3.9 x 10-6.
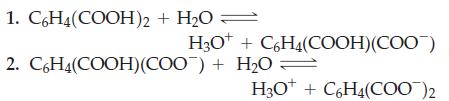
What are the pH values of the following aqueous solutions:
(a) 0.350 M potassium hydrogen orthophthalate;
(b) A solution containing 36.35 g potassium ortho-phthalate per liter?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





