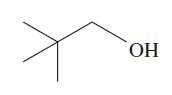
The molecule in the next column is 2,2-dimethylpropan-1-ol, a primary alcohol. Because no H atoms are bonded
Question:
The molecule in the next column is 2,2-dimethylpropan-1-ol, a primary alcohol. Because no H atoms are bonded to the β carbon atom in this molecule, dehydration seems unlikely. However, when 2,2-dimethylpropan-1-ol is heated with an acid catalyst, 2-methylbut-2-ene is obtained. Suggest a mechanism that shows how 2-methylbut-2-ene is formed.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





