The phosphorus isotope 32 P is used in biochemical studies to determine the pathways of phosphorus atoms
Question:
The phosphorus isotope 32P is used in biochemical studies to determine the pathways of phosphorus atoms in living organisms. Its presence is detected through its emission of β– particles.
(a) What is the decay constant for 32P, expressed in the unit s–1?
(b) What is the activity of a 1.00 mg sample of 32P (that is, how many atoms disintegrate per second)?
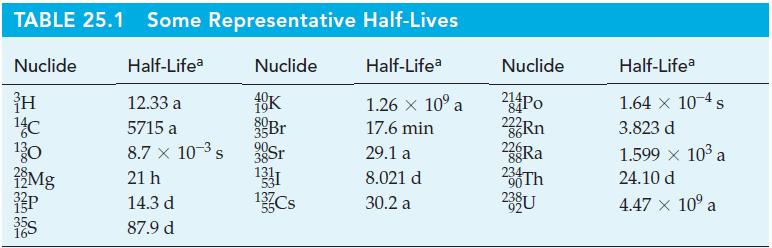
(c) Approximately what mass of 32P will remain in the original 1.00 mg sample after 57 days? (See Table 25.1.)
(d) What will be the rate of radioactive decay after 57 days?
Table 25.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





