The reaction 2 NO + 2 H 2 N 2 + 2 H 2 O is
Question:
The reaction 2 NO + 2 H2 → N2 + 2 H2O is second order in [NO] and first order in [H2]. A three-step mechanism has been proposed. The first, fast step is the elementary process given as equation (20.25). The third step, also fast, is N2O + H2 → N2 + H2O. Propose an entire three-step mechanism, and show that it conforms to the experimentally determined reaction order.
Eq. 20.25
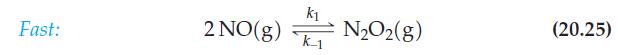
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





