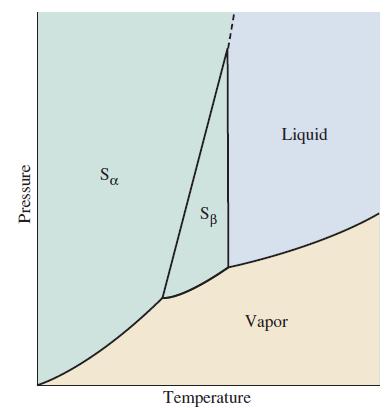
The sketch is a portion of the phase diagram for the element sulfur. The transition between solid
Question:
The sketch is a portion of the phase diagram for the element sulfur. The transition between solid orthorhombic (Sα) and solid monoclinic (Sβ) sulfur, in the presence of sulfur vapor, is at 95.3 °C. The triple point involving monoclinic sulfur, liquid sulfur, and sulfur vapor is at 119 °C.
(a) How would you modify the phase diagram to represent the melting of orthorhombic sulfur that is sometimes observed at 113 °C? What would the phase diagram look like if the monoclinic sulfur did not form?
(b) Account for the observation that if a sample of rhombic sulfur is melted at 113 °C and then heated, the liquid sulfur freezes at 119 °C upon cooling.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette




