The standard enthalpy of formation of gaseous H 2 O at 298.15 K is -241.82 kJ mol
Question:
The standard enthalpy of formation of gaseous H2O at 298.15 K is -241.82 kJ mol-1. Using the ideas contained in Figure 7-16, estimate its value at 100.0 °C given the following values of the molar heat capacities at constant pressure: H2O(g): 33.58 J K-1 mol-1; H2(g): 28.84 J K-1 mol-1; O2(g): 29.37 J K-1 mol-1. Assume the heat capacities are independent of temperature.
Figure 7-16
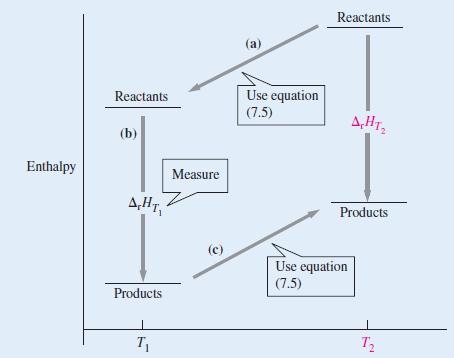
Transcribed Image Text:
Enthalpy Reactants (b) A.HT, Products T₁ Measure (c) (a) Use equation (7.5) Reactants A,HT₂2 Products Use equation (7.5) T₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
To estimate the standard enthalpy of formation of gaseous H2O at 1000 C 37315 K using the ideas cont...View the full answer

Answered By

Nithesh M
This is Vijaya Chandra, I was graduated from JNTU Engineering College in Mechanical Engineering. I have always shown a keen interest in physics, which is evident from his exceptional academic record in the subject. During my academic years, I was excelled in physics, consistently achieving high grades and performing well in exams. In addition to academic background, I worked as a tutor for 5 years in local village by helping students of all ages and skill levels achieve their goals. As a tutor, I have taught students of all classes for a period of five years, including graduate-level physics and have a deep understanding of the subject and passion for teaching has allowed me to develop unique teaching methods that make complex concepts easy to understand. I am comfortable working with students one-on-one or in small groups, and I am dedicated to helping them build confidence and succeed in their studies. Outside tutoring, I enjoyed playing cricket and surfing internet browser.
0.00
0 Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The molar heat capacity of ethane is represented in the temperature range 298 K to 400 K by the empirical expression Cp,m/ (J K-1 mol-1) = 14.73 + 0.1272(T/K). The corresponding expressions for C(s)...
-
The standard enthalpy of formation of the metallocene bis (benzene) chromium was measured in a calorimeter. It was found for the reaction Cr (C6H6)2(s) Cr(s) + 2 C6H6 (g) that Uo (583 K) = +8.0 kJ...
-
The head of a strike anywhere match contains tetraphosphorus trisulfide, P4S3. In an experiment, a student burned this compound in an excess of oxygen and found that it evolved 3651 kJ of heat per...
-
Secondary xylem and phloem in dicot stem are produced by (a) Phellogen (b) Apical meristems (c) Axillary meristems (d) Vascular cambium
-
During the current month, a company that uses a job order cost accounting system incurred a monthly factory payroll of $75,000, paid in cash. Of this amount, $29,000 is classified as indirect labor...
-
The sales manager at Grossmieller Importers in New York City needs to determine a monthly forecast for the number of mens golf sweaters that will be sold so that he can order an appropriate amount of...
-
What is capital rationing? AppendixLO1
-
Milani, Inc., acquired 10 percent of Seida Corporation on January 1, 2017, for $190,000 and appropriately accounted for the investment using the fair-value method. On January 1, 2018, Milani...
-
To prepare budgets, which of the following answers represents a correct sequence? Multiple Choice Sales budget - raw materials purchases budget - production budget - budgeted income statement...
-
Among its other features, the MyTVLab website allows customers to purchase MyTVLab LifeStyles merchandise online. To handle payment processing, the management of MyTVLab has contracted with the...
-
How much heat is required to convert 10.0 g of ice at -5.0 C to steam at 100.0 C? The temperature dependent constant-pressure specific heat capacity of ice is c p (T)/(kJ kg -1 K -1 ) = 1.0187T -...
-
The oxidation of NH 3 (g) to NO(g) in the Ostwald process must be very carefully controlled in terms of temperature, pressure, and contact time with the catalyst. This is because the oxidation of NH...
-
Archibald Ltd manufactures and sells one product. Its budgeted profit statement for the first month of trading is as follows: The budget was prepared using absorption costing principles. If budgeted...
-
Oswego Clay Pipe Company provides services of $ 5 0 , 0 0 0 to Southeast Water District # 4 5 on April 1 2 of the current year with terms 1 / 1 5 , n / 6 0 . What would Oswego record on April 1 2 ?...
-
Assume the following excerpts from a company's balance sheet: Property, plant, and equipment Beginning Balance $3,500,000 Ending Balance $3,700,000 $1,100,000 $800,000 Long-term investments During...
-
On January 1, 2021, Bonita Corp. had472,000shares of common stock outstanding. During 2021, it had the following transactions that affected the Common Stock account. February 1 Issued 125,000shares...
-
The pipe assembly is mounted vertically as shown in (Figure 1). Part A Figure 200 mm 80 mm- 1.2 m Determine the pressure at A if the velocity of the water ejected from B is 0.77 m/s. Express your...
-
Suppose you were asked to estimate the probability that your coin came up "Heads" from your observations. Provide such an estimate including a 90% confidence interval. Explain your calculations.
-
In which figures showing setups do you find the following being used as a workholding device? a. Three-jaw chuck b. Collet c. Faceplate d. Four-jaw chuck?
-
A new car sold for $31,000. If the vehicle loses 15% of its value each year, how much will it be worth after 10 years?
-
Gross Profit Method Presented below is information related to Jerrold Corporation for the current year. Compute the ending inventory , assuming that (a) Gross profit is 40% of sales; (b) Gross profit...
-
Retail Inventory Method Presented below is information related to McKenna Company. (a) Compute the ending inventory at retail. (b) Compute a cost-to-retail percentage (round to two decimals) under...
-
Retail Inventory Method Presented below is information related to Kuchinsky Company. Compute the inventory by the conventional retail inventory method. Retail Cost Beginning inventory Purchases...
-
Question 1 (3 points) Managerial accounting prepares historical information. O True O False Question 2 (3 points) Financial accounting prepares historical information. True O O False
-
Problem 5.30 Your answer is partially correct. Try again. Congress and the president have decided to increase the federal tax rate in an effort to reduce the budget deficit. Suppose that Patricia...
-
1)The Mace Company fails to record these Journal Entries Accrued Expense $30 Expiration of Prepaid Insurance $40 Paid Cash to purchase Supplies $8 Required: Determine the net effect of these errors...

Study smarter with the SolutionInn App


