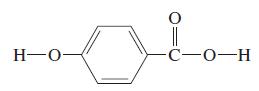
The structural formula shown is para-hydroxybenzoic acid, a weak diprotic acid used as a food preservative. Titration
Question:
The structural formula shown is para-hydroxybenzoic acid, a weak diprotic acid used as a food preservative. Titration of 25.00 mL of a dilute aqueous solution of this acid requires 16.24 mL of 0.0200 M NaOH to reach the first equivalence point. The measured pH after the addition of 8.12 mL of the base is 4.57; after 16.24 mL, the pH is 7.02. Determine the values of pKa1 and pKa2 of para-hydroxybenzoic acid and the pH values for the two equivalence points in the titration.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





