Two electrochemical cells are connected as shown. (a) Do electrons flow in the direction of the red
Question:
Two electrochemical cells are connected as shown.
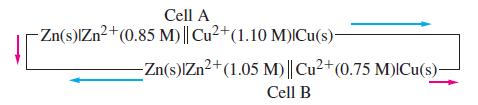
(a) Do electrons flow in the direction of the red arrows or the blue arrows?
(b) What are the ion concentrations in the half-cells at the point at which current ceases to flow?
Transcribed Image Text:
Cell A -Zn(s)IZn²+ (0.85 M)|| Cu2+(1.10 M) Cu(s)- -Zn(s)IZn²+ (1.05 M)|| Cu²+ (0.75 M)|Cu(s)- Cell B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Analyze The two cells differ only in their ion concentrations which means that they hav...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Tandex Corporation reported $175,000 of salaries expense on its income statement. The beginning and ending salaries payable amounts are $55,000 and $60,000, respectively. If Tandex uses the direct...
-
Two members, each consisting of straight and 8.4-in.-radius quarter circle portions, are connected as shown and support a 120-lb load at D. Determine the internal forces at point K. 42 in. 84 in. 4.2...
-
Two tanks are connected as shown in Fig. P3.49, both containing water. Tank A is at 200 kPa, v = 0.5 m3/kg, VA = 1 m3 and tank B contains 3.5 kg at 0.5 MPa, 400C. The valve is now opened and the two...
-
In Exercises 13 through 20, use the chain rule to compute the derivative dy/dx for the given value of x. y ==;u= u 3 - 1 for x 2
-
Review the multistep income statement presented in Exhibits 5-3 and 5-4. In your group, discuss how this form of the income statement meets each of these qualitative characteristics of accounting...
-
Respond to each of the following questions using this partially completed one-way ANOVA table: a. How many different populations are being considered in this analysis? b. Fill in the ANOVA table with...
-
What is competitive advantage? LO.1
-
In Problem 9, the first-order interactions between Z and the predictor variables X1, X3, and X4 were not included in the model selection process. Investigate whether this was appropriate, as follows...
-
Explain the difference between a call option and a put option. Explain the difference between an American option and European option. Find the value of a call option using the binomial option pricing...
-
The cell diagram for an electrochemical cell is written as Write the equations for the half-cell reactions that occur at the electrodes. Balance the overall cell reaction. Ni(s)...
-
(A) Write the overall equation for the redox reaction that occurs in the voltaic cell (B) Draw a voltaic cell in which silver ion is displaced from solution by aluminum metal. Label the cathode, the...
-
Red Cross laboratory The Red Cross hospital has a laboratory where blood tests are conducted. Monthly costs are given, as well as the number of blood monsters (samples) examined each month (see data,...
-
Drs. Draper and Keys run a partnership family medical practice in Brownsville, Texas. While the practice is profitable, both physicians are making payments on heavy debt loads for student loans that...
-
Sweetlip Ltd and Warehou Ltd are two family-owned flax-producing companies in New Zealand. Sweetlip Ltd is owned by the Wood family and the Bradbury family owns Warehou Ltd. The Wood family has only...
-
Small Sample Weights of M&M plain candies are normally distributed. Twelve M&M plain candies are randomly selected and weighed, and then the mean of this sample is calculated. Is it correct to...
-
Swain Athletic Gear (SAG) operates six retail outlets in a large Midwest city. One is in the center of the city on Cornwall Street and the others are scattered around the perimeter of the city....
-
The Tokyo Olympics After watching How the Tokyo Olympics Became the Most Expensive Summer Game Ever video answer the following questions. * * The numbers can be made up . I just need help with an...
-
Assume that on January 2, 2016, Drake-Neiman purchased fixtures for $8,000 cash, expecting the fixtures to remain in service for five years. Drake-Neiman has depreciated the fixtures on a...
-
Flicker, Inc., a closely held corporation, acquired a passive activity this year. Gross income from operations of the activity was $160,000. Operating expenses, not including depreciation, were...
-
Pickeril Inc. issues a $600,000, 10%, 10-year mortgage note on December 31, 2010, to obtain financing for a new building. The terms provide for semiannual installment payments of $48,145. Prepare the...
-
Prepare the journal entries that the lessee should make to record the following transactions. 1. The lessee makes a lease payment of $80,000 to the less or in an operating lease transaction. 2....
-
Presented below are long-term liability items for Molini Company at December 31, 2010. Prepare the long-term liabilities section of the balance sheet for Molini Company. Bonds payable, due 2012 ......
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...
-
John has to choose between Project A and Project B, which are mutually exclusive. Project A has an initial cost of $30,000 and an internal rate of return of 16 percent. Project B has an initial cost...
-
Complete the table below, for the above transactions

Study smarter with the SolutionInn App


