Two equations can be written for the dissolution of Mg(OH) 2 (s) in acidic solution. (a) Explain
Question:
Two equations can be written for the dissolution of Mg(OH)2(s) in acidic solution.
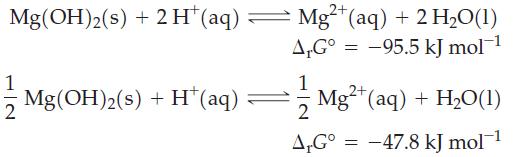
(a) Explain why these two equations have different ΔrG° values.
(b) Will K for these two equations be the same or different? Explain.
Transcribed Image Text:
2+ Mg(OH)2(s) + 2H* (aq) — Mg²+ (aq) + 2 H₂O(1) A.G° -95.5 kJ mol-¹ 1 Mg(OH)2(s) + H* (aq) 1 2 A,Gº = 2+ Mg²+ (aq) + H₂O(1) -47.8 kJ mol-¹ =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a The two equations have different rG values because they represent different paths for the same chemical reaction The first equation shows the direct ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
In Section 11.3.1, we found the least squares estimators of α and β by a two-stage minimization. This minimization can also be done using partial derivatives. (a) Compute...
-
Water flows between the North American Great Lakes as depicted in Fig. P13.12. Based on mass balances, the following differential equations can be written for the concentrations in each of the lakes...
-
The schematic diagram of the solution process as the net sum of three steps in Figure 13.4 does not show the relative magnitudes of the three components because these will vary from case to case. For...
-
At the current year-end, a company shows the following unadjusted balances for selected accounts. a. After an analysis of future sales discounts, the company estimates that the Allowance for Sales...
-
The controller of Trenshaw Company wants to improve the companys control system by preparing a month-by-month cash budget. The following information is for the month ending July 31, 2014. June 30,...
-
A \(1.0-\mathrm{cm}\)-tall object is \(60 \mathrm{~cm}\) in front of a diverging lens that has a \(-30 \mathrm{~cm}\) focal length. Calculate the image position and height.
-
Describe the two techniques for knowledge elicitation via the use of stories.
-
You are evaluating audit results for current assets in the audit of Quicky Plumbing Co. You set the preliminary judgment about materiality for current assets at $15,000 for overstatements and at...
-
PLEASE HELP ASAP Hello, I need help with (e) (f) (g) and (h) but I'll put the full question for context. Here's the question PLEASE ONLY ANSWER (e) (f) (g) and (h) Bob is the owner of a small grocery...
-
Currently, CO 2 is being studied as a source of carbon atoms for synthesizing organic compounds. One possible reaction involves the conversion of CO 2 to methanol, CH 3 OH. With the aid of data from...
-
For the reaction 2 SO 2 (g) + O 2 (g) 2 SO 3 (g), Kc = 2.8 x 10 2 M -1 at 1000 K. (a) What is r G at 1000 K? (b) If 0.40 mol SO 2 0.18 mol O 2 , and 0.72 mol SO 3 are mixed in a 2.50 L flask at...
-
Explain the concept of the Sharpe performance measure.
-
Nike Company has hired a consultant to propose a way to increase the company\'s revenues. The consultant has evaluated two mutually exclusive projects with the following information provided for...
-
What are the most effective way to manage routine and catastrophic disasters, and are they different?
-
The Wall Street Journal reported that of taxpayers with adjusted gross incomes between and itemized deductions on their federal income tax return. The mean amount of deductions for this population of...
-
1) Why do you believe that in recent years PE sponsors have increasingly chosen to buy debt in their distressed LBOs? 2) What are the pros and cons of this investment strategy? 3) What issues are...
-
Paper Street Soap Company Ltd conducts a business that makes luxury soaps. It operates a factory in Oyster Bay near Sydney. The factory contains a large amount of equipment that is used in the...
-
Find the input impedance of the circuit in Fig. 13.103. /6 102 862 -j20 i2
-
Design an experiment to demonstrate that RNA transcripts are synthesized in the nucleus of eukaryotes and are subsequently transported to the cytoplasm.
-
Weighted-average method, assigning costs (continuation of 17-19). For the data in Exercise 17-19, summarize total costs to account for, calculate cost per equivalent unit for direct materials and...
-
FIFO method, equivalent units. Refer to the information in Exercise 17-19. Suppose the Assembly Division at Fenton Watches, Inc., uses the FIFD method of process costing instead of the...
-
FIFO method, assigning costs (continuation of 11-21). For the data in Exercise 17-19, use the FIFO method to summarize total costs to account for, calculate cost per equivalent unit for direct...
-
If the month-end bank statement shows a balance of $75,000, outstanding checks are $54,000, a deposit of $15,000 was in transit at month end, and a check for $4,000 was erroneously charged by the...
-
SECTION A [100 MARKS] Answer ALL questions in this section. QUESTION 1 Explain the difference between financial and management accounting.
-
If Donald obtained a business loan of $270,000.00 at 5.34% compounded semi- annually, how much should he pay at the end of every 6 months to clear the loan in 25 years? Round to the nearest cent

Study smarter with the SolutionInn App


