Under what temperature conditions would the following reactions occur spontaneously? (a) 2 NH4NO3(s) - (b) I2(g) 2
Question:
Under what temperature conditions would the following reactions occur spontaneously?
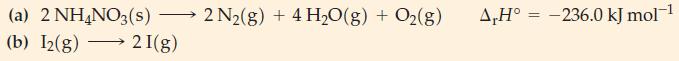
Transcribed Image Text:
(a) 2 NH4NO3(s) - (b) I2(g) 2 I(g) 2 N₂(g) + 4 H₂O(g) + O2(g) A,H° -236.0 kJ mol-¹ =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (6 reviews)
Analyze a The reaction is exothermic and in Example 132a we concluded that S 0 because large quantit...View the full answer

Answered By

Sarfraz gull
have strong entrepreneurial and analytical skills which ensure quality tutoring and mentoring in your international business and management disciplines. Over last 3 years, I have expertise in the areas of Financial Planning, Business Management, Accounting, Finance, Corporate Finance, International Business, Human Resource Management, Entrepreneurship, Marketing, E-commerce, Social Media Marketing, and Supply Chain Management.
Over the years, I have been working as a business tutor and mentor for more than 3 years. Apart from tutoring online I have rich experience of working in multinational. I have worked on business management to project management.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(A) Which of the four cases in Table 13.3 would apply to each of the following reactions? (B) Under what temperature conditions would the following reactions occur spontaneously? (a) The...
-
Which of the following reactions occur spontaneously as written, with the production of a measurable electric current? a. I 2 + NaBr Br 2 + 2NaI b. Li + NaCl LiCl + Na c. Li + + Na + NaLi d. Ag +...
-
Using data from Appendix 4, calculate ÎHo, ÎSo, and DGo for the following reactions that produce acetic acid: Which reaction would you choose as a commercial method for producing acetic...
-
As mentioned in Section 5.6, Sainte-Venants principle will allow particular boundary conditions to be replaced by their statically equivalent resultant. For problems (b), (c), (d),and (f) in Exercise...
-
Edington Electronics Inc. produces and sells two models of pocket calculators, XQ-103 and XQ-104. The calculators sell for $15 and $25, respectively. Because of the intense competition Edington...
-
When your hands are cold, you can rub them together to warm them. Explain the energy transformations that make this possible.
-
Distinguish between KM foundation and KM solutions. What are the components of KM foundation and KM solutions?
-
Prepare any necessary adjusting entries at December 31, 2013, for Maxum Companys year- end financial statements for each of the following separate transactions and events. 1. Employees earn vacation...
-
1. Calculate Beta for Asset H (Just follow the steps). STEP 1: Get the average returns for both the asset and the market Year 2004 2007 Returns Asset Market 0.15 0.1 0.125 0.07 0.115 0.08 -0.02 0...
-
Arrange the entropy changes of the following processes, all at 25 C, in the expected order of increasing S, and explain your reasoning: (a) HO(1, 1 bar) (b) CO (s, 1 bar) (c) HO(1, 1 bar) 111 HO(g, 1...
-
Indicate whether each of the following changes represents an increase or a decrease in entropy in a system, and explain your reasoning: (a) The freezing of ethanol; (b) The sublimation of dry ice;...
-
Use the given figure, translation vectors v and w, and reflection lines l and m to construct the indicated glide reflections. Show the triangle in the positions both before and after the glide...
-
a) What CSR did your organization do - how did it improve your organization's image? b) If your organization did not do any CSR, as the boss, what CSR activities would you suggest doing and why?
-
Do you believe NIL promotes "love of the game," or does it make college sports more about money and business? What are the most significant positive and negative effects of NIL, in your opinion? What...
-
Even well-managed organizations do not always work as efficiently and effectively as management would like. At Hewlett-Packard (HP), billions of dollars of product are being shipped - from computers...
-
Recognition is a very important element of volunteer management. Do you know someone who has done amazing volunteer work for a good cause? Wouldn't it be nice to thank them with an award! Take a look...
-
What do Financial Planners do? Would you consider hiring a Financial Planner? How important are ethics when working with a financial planning professional? Explain the concept of return on...
-
Find I1 and I2 in the circuit of Fig. 13.90. Calculate the power absorbed by the 4- Ω resistor. 112 5 3630 Vi, 12) 452 2
-
Suppose the index goes to 18 percent in year 5. What is the effective cost of the unrestricted ARM?
-
Backflush, two trigger points, completion of production and sale (continuation of 20-33). Assume the same facts as in Problem 20-33 except now there are only two trigger points: the completion of...
-
Lean Accounting. Flexible Security Devices (FSD) has introduced a just-in-time production process and is considering the adoption of lean accounting principles to support its new production...
-
Backflushing. The following conversation occurred between Brian Richardson, plant manager at Glendale Engineering, and Charles Cheng, plant controller. Glendale manufactures automotive component...
-
You make 24 deposits of $504 at the beginning of each month into a bank account. At the end of the 24th month, you will have $12,800 in your account. If the bank compounds interest monthly, what...
-
Investment banks act as dealers and are major investors in treasury securities T OR F
-
Suppose an investment is equally likely to have a 42% return or a -20% return. The total volatility of returns is closest to: Select one: a. 9.61% b. 43.84% c. 21.92% d. 31.00%

Study smarter with the SolutionInn App


