Use 1 x 10 -13 cm as the approximate diameter of the spherical nucleus of the hydrogen-1
Question:
Use 1 x 10-13 cm as the approximate diameter of the spherical nucleus of the hydrogen-1 atom, together with data from Table 2.1, to estimate the density of matter in a proton.
Table 2.1
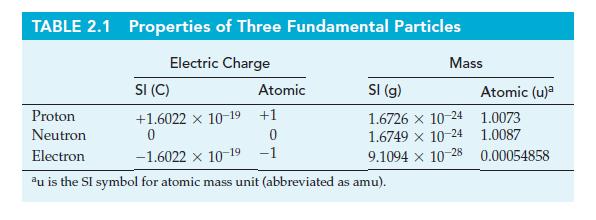
Transcribed Image Text:
TABLE 2.1 Properties of Three Fundamental Particles Electric Charge Atomic Mass SI (C) Proton +1.6022 x 10-19 +1 Neutron 0 0 Electron -1.6022 × 10-19 -1 au is the SI symbol for atomic mass unit (abbreviated as amu). SI (g) 1.6726 x 10-24 1.6749 × 10-24 9.1094 x 10-28 Atomic (u)² 1.0073 1.0087 0.00054858
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
Density d massg volume gcm mass g proton 16726x1...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The hydrogen atom is composed of one proton in the nucleus and one electron, which moves about the nucleus. In the quantum theory of atomic structure, it is assumed that the electron does not move in...
-
The large magnetic fields used in MRI can produce forces on electric currents within the human body. This effect has been proposed as a possible method for imaging biocurrents flowing in the body,...
-
An atom has a very small nucleus surrounded by an electron cloud. Figure 2.1 represents the nucleus with a diameter of about 2 mm and describes the electron cloud as extending over 200 m. If the...
-
Each of the following passages may be plausibly criticized by some who conclude that it contains a fallacy, but each may be defended by some who deny that the argument is fallacious. Discuss the...
-
a. A vacant lot acquired for $75,000 is sold for $145,000 in cash. What is the effect of the sale on the total amount of the sellers? (1) Assets (2) Liabilities (3) Owner's equity? b. Assume that the...
-
For each of the following examples of data, determine the type. a. The number of miles joggers run per week b. The starting salaries of graduates of MBA programs c. The months in which a firms...
-
Q5 How do value chains determine business processes and information systems?
-
Susan Knoll is an attorney in Los Angeles. Knoll uses the direct write-off method to account for uncollectible receivables. At January 31, 2016, Knolls accounts receivable totaled $18,000. During...
-
It has been evidenced that a growing number of Australian organizations offering support to the indigenous people in responding to the COVID -19 pandemic. What are the motivations for an Australian...
-
Fluorine has a single atomic species, 19 F. Determine the atomic mass of 19 F. by summing the masses of its protons, neutrons, and electrons, and compare your results with the value listed on the...
-
William Prout (1815) proposed that all other atoms are built up of hydrogen atoms, suggesting that all elements should have integral atomic masses based on an atomic mass of one for hydrogen. This...
-
Examine these extracts from Moonshine Companys income statement and balance sheet for the previous three years: Now calculate the ratio of accounts receivable as a percentage of sales for Years 1, 2,...
-
Mark Gold opened Gold Roofing Service on April 1. Transactions for April are as follows: 1 Gold contributed \(\$ 15,000\) of his personal funds in exchange for common stock to begin the business. 2...
-
n1 = 20, n2 = 30, S = 400, H1: m1 < m2. Exercises 57 present sample sizes and the sum of ranks for the rank-sum test. Compute S, S, and the value of the test statistic z. Then find the P-value.
-
The adjusted trial balance section of Menlo Company's worksheet shows a \(\$ 1,500\) debit balance in utility expense. At the end of the accounting period the accounting manager accrues an additional...
-
Identify each of the 10 amount columns of the worksheet and indicate to which column the adjusted balance of the following accounts would be extended: a. Accounts Receivable b. Accumulated...
-
Using the data from Table 3.3, show the effect on world output if each country moved toward specialization in the production of its comparative-disadvantage good. TABLE 3.3 Comparative Advantage as a...
-
Which would react more rapidly with Cl2 + FeCl3, m-xylene or p-xylene? Explain.
-
Review Exhibit 11.4. Analyze each product on the graph according to the characteristics that influence the rate of adoption. For example, what can you conclude from the data about the relative...
-
What are two potential tests that can be conducted to verify the CAPM? What are the results of such tests? What is rolls critique of CAPM tests?
-
Briefly explain the difference between the CAPM and the arbitrage pricing theory (APT).
-
Suppose you are given the following information. The beta of company, bi, is 0.9, the risk free rate, rRF, is 6.8%, and the expected market premium, rM-rRF, is 6.3%. Because your company is larger...
-
Following is a series of independent cases . In each situation, indicate the cash distribution to be made to partners at the end of the liquidation process. Unless otherwise stated, assume that all...
-
ASSETS LIABILITES AND EQUITY TII TTI TIT TTT EXPENSES REVENUES Inceresumman T T
-
. In explain the problem Distinguish between a meteor, a meteoroid, a meteorite, an asteroid, and a comet

Study smarter with the SolutionInn App


