Use both the ideal gas equation and the van der Waals equation to calculate the pressure exerted
Question:
Use both the ideal gas equation and the van der Waals equation to calculate the pressure exerted by 1.50 mol of SO2(g) when it is confined at 298 K to a volume of
(a) 100.0 L;
(b) 50.0 L;
(c) 20.0 L;
(d) 10.0 L.
Under which of these conditions is the pressure calculated with the ideal gas equation within a few percent of that calculated with the van der Waals equation? Use values of a and b from Table 6.5.
Table 6.5
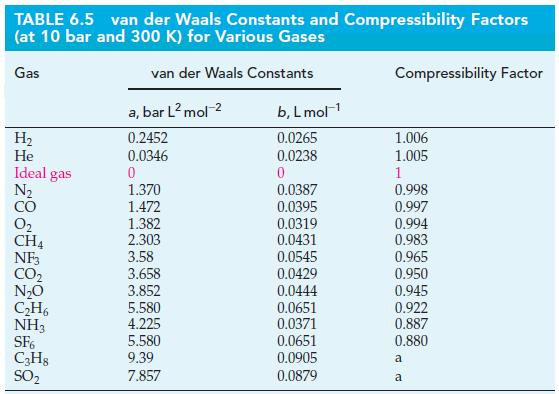
Transcribed Image Text:
TABLE 6.5 van der Waals Constants and Compressibility Factors (at 10 bar and 300 K) for Various Gases Gas van der Waals Constants H₂ He Ideal gas N₂ CO N₂O C₂H6 NH3 SF6 C3H8 SO₂ a, bar L² mol-² 0.2452 0.0346 0 1.370 1.472 1.382 2.303 3.58 3.658 3.852 5.580 4.225 5.580 9.39 7.857 b, L mol-¹ 0.0265 0.0238 0 0.0387 0.0395 0.0319 0.0431 0.0545 0.0429 0.0444 0.0651 0.0371 0.0651 0.0905 0.0879 Compressibility Factor 1.006 1.005 1 0.998 0.997 0.994 0.983 0.965 0.950 0.945 0.922 0.887 0.880 a a
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Two distillation columns are used to produce the products indicated in Figure 9.19. Establish the type of condenser and an operating pressure for each column for the: (a) Direct sequence (C 2 / C 3...
-
m) Muffin Megabucks is considering two different savings plans. The first plan would have her deposit $500 every six months, and she would receive interest at a 7 percent annual rate, compounded...
-
Refer to Example 6-17. Recalculate the pressure of Cl 2 (g) by using both the ideal gas equation and the van der Waals equation at the temperatures (a) 100 C; (b) 200 C; (c) 400 C. From the results,...
-
Suppose that the fixed cost for a product is $400 and the break-even quantity is 80. Find the marginal profit (the slope of the linear profit function).
-
Contrast the Project Plan, and the Work Breakdown Structure.
-
On August 1, 2014, Treadwell Co. received $10,500 for the rent of land for 12 months. Journalize the adjusting entry required for unearned rent on December 31, 2014.
-
Learn ways to improve your communication skills
-
1. Based on the information given, summarize the method(s) BMW intends to use for determining incentive pay. Are these rewards for individual, group, and/or company performance? 2. Explain BMWs claim...
-
Need help asap! will upvote! V. Performance Over Time A. Analyze the performance of the TESLA over time. What financial strengths and weaknesses does this company have? Consider addressing the free...
-
Use the value of the van der Waals constant b for He(g) given in Table 6.5, to estimate the radius, r, of a single helium atom. Give your answer in picometers. Table 6.5 TABLE 6.5 van der Waals...
-
The molar mass of radon gas was first estimated by comparing its diffusion rate with that of mercury vapor, Hg(g). What is the molar mass of radon if mercury vapor diffuses 1.082 times as fast as...
-
The Ka for dichloroacetic acid is 3.32 x 102. Approximately what percentage of the acid is dissociated in a 0.10M aqueous solution?
-
The TechTeach Company produces and sells 7,000 modular computer desks per year at a selling price of $750 each. Its current production equipment, purchased for $1,950,000 and with a 5-year useful...
-
1. The following data are available for JURIS DOCTOR CORP: Purchased raw materials from supplier amounting to P 40,000 on account.; During the month, raw materials costing P 30,000 were issued to...
-
The following financial information is available for Concord Corporation. (in millions) 2025 2024 Average common stockholders' equity $2,500 $2,600 Dividends declared for common stockholders 305 594...
-
Vecton's Bakery manufactures apple turnovers that passes through 4 sequential processes. Production data for February for Department 4 of the operation is as follows: Production data Units Opening...
-
write a code in java where we apply the sets and subsets to obtain functions as results. Let A= {1,2,3,4}, B={5,6,7,0}, C={8,9,10,11} and f: AB g:BC h: BC, all function are 1 to 1 a) Form the...
-
For a closed-subshell configuration, (a) Show that the double sum in (11.80) equals Where the Coulomb and exchange integrals are defined in terms of the n/2 different spatial orbitals Ï; (b) Use...
-
Teasdale Inc. manufactures and sells commercial and residential security equipment. The comparative unclassified balance sheets for December 31, 2015 and 2014 are provided below. Selected missing...
-
What is meant by accounting symmetry between the entries recorded by the debtor and creditor in a troubled debt restructuring involving a modification of terms? In what ways is the accounting for...
-
Under what circumstances a transaction would be recorded as a troubled-debt restructuring by only one of the two parties to the transaction?
-
Whiteside Corporation issues $500,000 of 9% bonds, due in 10 years, with interest payable semiannually. At the time of issue, the market rate for such bonds is 10%. Compute the issue price of the...
-
a. Performed $8,200 of services on account. b. Collected $5,600 cash on accounts receivable. c. Paid $1,450 cash in advance for an insurance policy. d. Paid $400 on accounts payable. e. Recorded the...
-
Question 3 Assume that the following financial ratios were computed from the 2017 financial statements of Florida Industries: Return on sales (profit margin) 0.29 Return on assets 0.17 Common equity...
-
Balsam Company has recently tried to improve its analysis for its manufacturing process. Units started into production equaled 25,000 and ending work in process equaled 12.500 units. Balsam had no...

Study smarter with the SolutionInn App


