Question: Use data from Figure 20-12 to determine the temperature at which t 1/2 for the first-order decomposition of N 2 O 5 in CCl 4
Use data from Figure 20-12 to determine the temperature at which t1/2 for the first-order decomposition of N2O5 in CCl4 is 2.00 h.
Figure 20-12
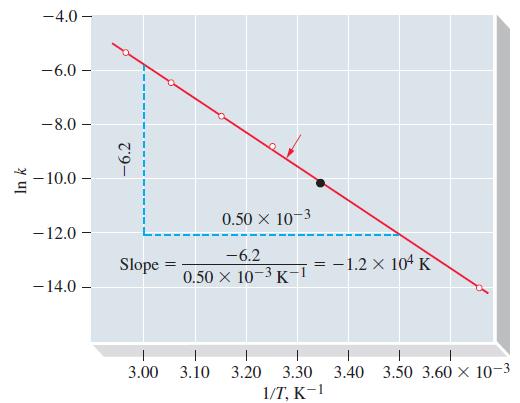
In k -4.0 - -6.0- -8.0 -10.0- -12.0 - - 14.0 -6.2 Slope = 3.00 0.50 10-3 -6.2 0.50 x 10-3K-1 3.10 3.20 3.30 1/T, K-1 -1.2 x 104 K 3.40 3.50 3.60 10-3
Step by Step Solution
3.33 Rating (159 Votes )
There are 3 Steps involved in it
Analyze First find the rate constant k corresponding to a 200 h halflife This can be done by using t... View full answer

Get step-by-step solutions from verified subject matter experts


