Use data from Table 12.5 to estimate (a) The boiling point of water in Santa Fe, New
Question:
Use data from Table 12.5 to estimate
(a) The boiling point of water in Santa Fe, New Mexico, if the prevailing atmospheric pressure is 640 mmHg;
(b) The prevailing atmospheric pressure at Lake Arrowhead, California, if the observed boiling point of water is 94 °C.
Table 12.5
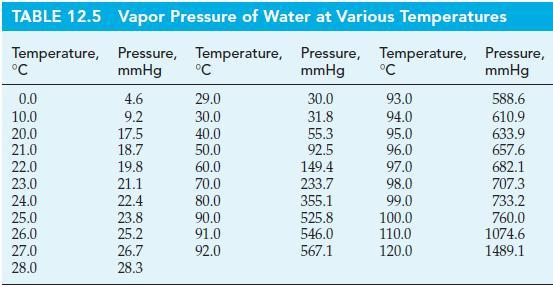
Transcribed Image Text:
TABLE 12.5 Temperature, °C 0.0 10.0 20.0 21.0 22.0 23.0 24.0 25.0 26.0 27.0 28.0 Vapor Pressure of Water at Various Temperatures Pressure, Temperature, Pressure, Temperature, mmHg mmHg 4.6 9.2 17.5 18.7 19.8 21.1 22.4 23.8 25.2 26.7 28.3 °C 29.0 30.0 40.0 50.0 60.0 70.0 80.0 90.0 91.0 92.0 30.0 31.8 55.3 92.5 149.4 233.7 355.1 525.8 546.0 567.1 °C 93.0 94.0 95.0 96.0 97.0 98.0 99.0 100.0 110.0 120.0 Pressure, mmHg 588.6 610.9 633.9 657.6 682.1 707.3 733.2 760.0 1074.6 1489.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a Since the boiling point of a liquid is the temperature at which its vapor pressure equal...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Create a simple video conferencing web app (P2P or WebRTC). Needs to handle more than one-on-one communication and should have option to screen share as well. (prefer not to use sockets if possible....
-
Perform the following computation on 8-bit numbers (the encoding is irrelevant here). Write down: i) the 8-bit result (include all leading zeros, if any). ii) the status of the flags: carry and...
-
Consider these historical returns for these three assets Domestic Foreign Other -0.9% 5.7% 19.7% 3.5% 5.3% 19.3% 10.7% -1.4% 18.7% 0.1% 0.4% 0.1% 9.6% 4.7% 17.6% 11.7% -0.9% -1.4% 11.3% 3.5% 0.4%...
-
Information sent electronically over the Internet affords little privacy. One of the reasons that cryptography is included in this text is because its importance is growing due to the increasing need...
-
Deitz is a publishing company with a number of different magazines and other publications. The company also owns a printing operation called Saira Press. The publications and the printing operation...
-
You are discussing physics homework with a friend while the two of you are riding your bikes. Distracted, you collide with a parked car. The car doesn't move. Your friend says, "Of course not! The...
-
Why do we say that understanding a story involves more than adding up the meanings of the sentences that make up the story? LO1
-
Annular flow with inner cylinder moving axially (see Fig. 2B.7), a cylindrical rod of diameter KR moves axially with velocity v0 along the axis of a cylindrical cavity of radius R as seen in the...
-
Securitie Ltd's common size statements of financial performance for 2017 and 2018 are presented below. All amounts are expressed as percentage of revenue. Revenue (all sales) Cost of sales Gross...
-
An 80.0 g piece of dry ice, CO 2 (s), is placed in a 0.500 L container, and the container is sealed. If this container is held at 25 C, what state(s) of matter must be present?
-
A sample of 113 L of helium gas at 1360 C and prevailing barometric pressure is passed through molten silver at the same temperature. The gas becomes saturated with silver vapor, and the liquid...
-
A positive point charge Q1 = 2.5 X 10-5 C is fixed at the origin of coordinates, and a negative charge Q2 = 5.0 X 10-6C is fixed to the x axis at x = + 2.0m. Find the location of the place(s) along...
-
THE SHRM Learning system provides several motivation theories that increase engagement. Which of the motivation theories most aligns your real world experience as personally motivating you and why?...
-
Leadership and management are two distinct yet complementary concepts within organizations. Leadership is about inspiring and influencing others towards a shared vision or goal, often focusing on...
-
Analyse the need and want(s) that led you to research products or services that would address the state of your imbalance. 2. Examine the internal and external sources of information by including...
-
What is an incident in which a famous person wore or used a product (not as part of a paid endorsement or ad) and it caused a buying frenzy. Explain how the manufacturer or service provider reacted
-
What is a "heavyweight project team" and how does it differ from the traditional approach used for organizing development projects at Eli Lilly?This consists of two issues:First, an evaluation of the...
-
A three-phase generator supplied 3.6 kVA at a power factor of 0.85 lagging. If 2500 W are delivered to the load and line losses are 80 W per phase, what are the losses in the generator?
-
A survey of 70 college freshmen asked whether students planned to take biology, chemistry, or physics during their first year. Use the diagram to answer each question. How many of the surveyed...
-
Cost allocation in hospitals, alternative allocation criteria. Dave Meltzer vacationed at Lake Tahoe last winter. Unfortunately, he broke his ankle while skiing and spent two days at the Sierra...
-
Allocating Costs to Divisions Gether Corporation manufactures appliances. It has four divisions: Refrigerator, Stove, Dishwasher, and Microwave oven. Each division is located in a different city and...
-
Cost allocation to divisions. Rembrandt Hotel & Casino is situated on beautiful Lake Tahoe in Nevada. The complex includes a 300-room hotel, a casino, and a restaurant. As Rembrandts new controller,...
-
do you think regulation helps prevent occurrence of accounting scandals? Or do you think it is positively related to future accounting scandals?
-
Suppose the rate of return on short-term government securities (perceived to be risk-free) is about 5%. Suppose also that the expected rate of return required by the market for a portfolio with a...
-
Year-to-date, Company O had earned a -2.40 percent return. During the same time period, Company V earned 8.3 percent and Company M earned 6.55 percent. If you have a portfolio made up of 10 percent...

Study smarter with the SolutionInn App


