Use the analyses of a bcc structure and the fcc structure in Exercise 146 to determine the
Question:
Use the analyses of a bcc structure and the fcc structure in Exercise 146 to determine the percent voids in the packing-of-spheres arrangement found in the fcc crystal structure.
Exercise 146
The fcc unit cell is a cube with atoms at each of the corners and in the center of each face, as shown here. Copper has the fcc crystal structure. Assume an atomic radius of 128 pm for a Cu atom.
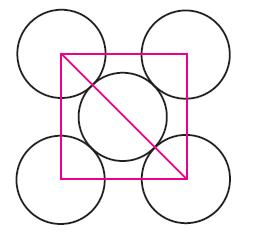
(a) What is the length of the unit cell of Cu?
(b) What is the volume of the unit cell?
(c) How many atoms belong to the unit cell?
(d) What percentage of the volume of the unit cell is occupied?
(e) What is the mass of a unit cell of copper?
(f) Calculate the density of copper.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





