Use the data here and in Table 7.2 to calculate f H of benzene, C 6
Question:
Use the data here and in Table 7.2 to calculate ΔfH° of benzene, C6H6(l).
![]()
Table 7.2
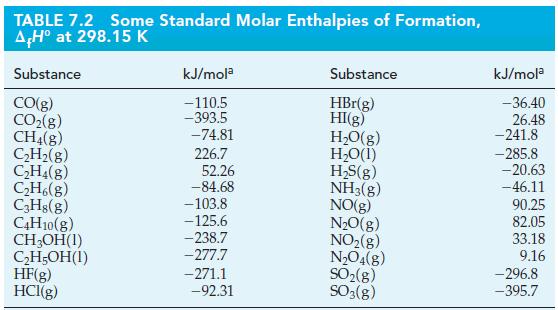
Transcribed Image Text:
2 C6H6(1) 15 O₂(g) 12 CO₂(g) + 6H₂O(1) A,H = -6535 kJ mol-1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Analyze We have a chemical equation and know the standard enthalpy of reaction We are asked ...View the full answer

Answered By

Usman Nasir
I did Master of Commerce in year 2009 and completed ACCA (Association of Chartered Certified Accountants) in year 2013. I have 10 years of practical experience inclusive of teaching and industry. Currently i am working in a multinational company as finance manager and serving as part time teacher in a university. I have been doing tutoring via many sites. I am very strong at solving numerical / theoretical scenario-based questions.
4.60+
16+ Reviews
28+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use the data here to draw the supply and demand curves for a hamburger. You do not have to share your drawings; just reference them to answer the questions. Imagine a hamburger has the following...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Consider benzene (C6H6) in the gas phase. (a) Write the reaction for breaking all the bonds in C6H6 (g), and use data in Appendix C to determine the enthalpy change for this reaction. (b) Write a...
-
Which of the following is not a strategic disadvantage of vertical integration? Vertical integration poses all kinds of capacity-matching problems (achieving the most efficient scale of operation for...
-
Read the chapter opener about Hood River Juice Company. David Ryan explained that purchasing apples year-round and processing them immediately reduces costs, and that his company blends juices to fit...
-
Consult Paragraphs 12 of Ethics Rule 102 (ET 102). Next, consider the roles of Ron Lomenzo and Lisa Taranto. Assume that these employees knew that the entries being proposed by Scott Sullivan were...
-
Review the case study. Discuss possible alternative approaches to the design and analysis of data. Discuss why the case study was an analytic study.
-
Temco Industries has developed a forecasting model that was used to forecast during a ten-month period. The forecasts and actual demand are shown as follows: Measure the accuracy of the forecast...
-
for Receivables P 8 . 8 A ( LO 2 ) AP Information on Hohenberger Company for 2 0 2 4 follows: 4 . Asume that Hohenberger Company decides to estimate its uncollectible accounts using the alliswance...
-
Calculate the source pressure required to deliver 13 x 10 6 standard cubic feet per day (SCFD) of CO to a customer 60 mi. away. By contract the pressure at the customer's site must be at least 300 lb...
-
The enthalpy of formation of formaldehyde is f H = -108.6 kJ/mol at 298.15 K. Write the chemical equation to which this value applies.
-
Let us apply equation (7.22) to calculate the standard enthalpy of combustion of ethane, C 2 H 6 (g), a component of natural gas. Eq. 7.22 A,H = [c x AHc + dx AHD +...] [ax AHA + bx AfHB +...] (7.22)...
-
Why might a loan application be a good place to look for evidence of hidden assets?
-
This week we learned about assessing competition. Watch the video the History of the Cola Wars and answer the following questions. Using the frameworks from the text and the online lesson, why is...
-
Prior to developing your training programs, you must analyze your organizational military needs, identify employee skills gaps based on performance, and have resources available to support training...
-
Describe specifically how your firm's culture lines up with the bullet points listed for that firm . For instance, if you believe your organization's strategy priority is creativity-driven , then...
-
You have recently taken over daycare center that was under substandard leadership. Currently, the staff is unmotivated, negative, and often absent from work. You notice that there is minimal...
-
Choose an organization from the industry of your choice to discuss, illustrate, and reflect deliberately on the following: Why is it important to distinguish between "group" and "team "? What kinds...
-
Why are the pitch and radius of the gullet between teeth on a broach of importance?
-
An investor sells a European call on a share for $4. The stock price is $47 and the strike price is $50. Under what circumstances does the investor make a profit? Under what circumstances will the...
-
Entries for Held-to-Maturity Securities on January 1, 2009, Roosevelt Company purchased 12% bonds, having a maturity value of $500,000, for $537,907.40. The bonds provide the bondholders with a 10%...
-
Entries for Available-for-Sale Securities assume the same information as in E17-3 except that the securities are classified as available-for-sale. The fair value of the bonds at December 31 of each...
-
Effective-Interest versus Straight-Line Bond Amortization on January 1, 2010, Morgan Company acquires $300,000 of Nicklaus, Inc., 9% bonds at a price of $278,384. The interest is payable each...
-
help! Problem 14-14 (Algo) 4 A supplier of instrument gauge clusters uses a kanban system to control material flow. The gauge cluster housings are transported four at a time. A fabrication center...
-
Structuring a Special-Order Problem Rabbit Foot Motors has been approached by a new customer with an offer to purchase 5,000 units of its hands-free, Wi-Fi-enabled automotive modelthe SMAKat a price...
-
For journal entries 1 through 6 identify the explanation that most closely describes it. A. To record this period's depreciation expense. B. To record accrued salaries expense. C. To record this...

Study smarter with the SolutionInn App


