What is the (a) Degree of ionization and (b) Percent ionization of trichloroacetic acid in a 0.035
Question:
What is the
(a) Degree of ionization and
(b) Percent ionization of trichloroacetic acid in a 0.035 M CCl3COOH solution?
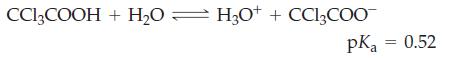
Transcribed Image Text:
CC13COOH + H₂0 ⇒ H3O+ + CCl₂COO pka 0.52 =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
To find the degree of ionization and percent ionization of trichloroacetic acid CCl3COOH in a 0035 M ...View the full answer

Answered By

Tobias sifuna
I am an individual who possesses a unique set of skills and qualities that make me well-suited for content and academic writing. I have a strong writing ability, allowing me to communicate ideas and arguments in a clear, concise, and effective manner. My writing is backed by extensive research skills, enabling me to gather information from credible sources to support my arguments. I also have critical thinking skills, which allow me to analyze information, draw informed conclusions, and present my arguments in a logical and convincing manner. Additionally, I have an eye for detail and the ability to carefully proofread my work, ensuring that it is free of errors and that all sources are properly cited. Time management skills are another key strength that allow me to meet deadlines and prioritize tasks effectively. Communication skills, including the ability to collaborate with others, including editors, peer reviewers, and subject matter experts, are also important qualities that I have. I am also adaptable, capable of writing on a variety of topics and adjusting my writing style and tone to meet the needs of different audiences and projects. Lastly, I am driven by a passion for writing, which continually drives me to improve my skills and produce high-quality work.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Derive the form of the Langmuir isotherm when an adsorbing species occupies two surface sites, i.e: Diagrams included in the Solution
-
Exploring the degree of ionization equation in Study Question 127: Calculate the degree of ionization, , for formic acid at the following concentrations: 0.0100 M, 0.0200 M, 0.0400 M, 0.100 M, 0.200...
-
What is the (a) Degree of ionization and (b) Percent ionization of propionic acid in a solution that is 0.45 M CH 3 CH 2 CO 2 H? CH3CHCOH + HO H3O+ + CH3CHCO pka = 4.89
-
Refer to the adjusted trial balance for Romney's Marketing Company in M4-8. Prepare the closing entry at the end of the current year. M4-8 Romney's Marketing Company has the following adjusted trial...
-
How can the firm use currency options to hedge foreign-currency exposures resulting from international transactions?
-
A Fortune study found that the variance in the number of vehicles owned or leased by subscribers to Fortune magazine is .94. Assume a sample of 12 subscribers to another magazine provided the...
-
5. A colleague suggests that a 6 percent growth rate is too low for revenue, profit, and cash flow growth beyond year 5. He suggests raising growth to 12 percent in the continuing-value period. If...
-
Parent Corporation owns 100% of Subsidiary Corporations single class of stock. Its adjusted basis for the stock is $175,000. After adopting a plan of liquidation, Subsidiary distributes the following...
-
Zoom Shoes Inc. has 115,000 shares of stock outstanding. GAS Running Company owns 35,000 shares of Zoom Shoes Inc. Which of the following is true? a.GAS Running Company is the subsidiary company....
-
Continuing the dilutions described in Example 16-4, should we expect the percent ionization to be 13% in 0.0010 M CH 3 COOH and 42% in 0.00010 M CH 3 COOH? Explain. Example 16-4 What is the percent...
-
What must be the molarity of an acetic acid solution if it has the same percent ionization as 0.100 M CH 3 CH 2 CO 2 H (propionic acid, K a = 1.3 x 10 -5 )?
-
Calculate the weight of the configuration in which 16 objects are distributed in the arrangement 0, 1, 2, 3, 8, 0, 0, 0, 0, 2.
-
Suppose a company bases its hourly rates on the number of customers per hour. The hourly rate the company charges is given by two functions where = g(2) 4, g(3) = 2, 9(4) = 3 and f(2) = 6, f(3) = 3,...
-
Which statements about insurance are true? 1- Insurance protects against the the worst-case scenario. All rational people want to buy insurance. 2- Insurance costs money, and therefore always...
-
need step by step instruction about creating this: in NX12 PART NAME: BRACKET ALL FILLETS R .313 ALL ROUNDS R .625 2X .500 1/500 2.875 9.500 4750 2875 $500 3.000 750 GENTERED IN OBJECT 2.375
-
8. Convert the angle - 7t from radian measure into degree measure. Show some work. 4
-
4. Variance Analysis. (CPA, adapted) The H. G. Company uses a standard cost system in accounting for the cost of one of its products. < The Budget is based on normal capacity of monthly production of...
-
Sudz Corporation has these accounts at December 31: Common Stock, $10 par, 5,000 shares issued, $50,000; Paid-in Capital in Excess of Par Value $22,000; Retained Earnings $42,000; and Treasury Stock,...
-
A superior criticized a sales manager for selling high-revenue, low-profit items instead of lower-revenue but higher-profit items. The sales manager responded, My income is based on commissions that...
-
A company reports the following: Net sales $560,000 Average accounts receivable (net) 40,000 Determine (a) The accounts receivable turnover and (b) The number of days sales in receivables. Round to...
-
A company reports the following: Net sales $600,000 Average accounts receivable (net) 60,000 Determine (a) The accounts receivable turnover and (b) The number of days sales in receivables. Round to...
-
A company reports the following: Cost of goods sold $510,000 Average inventory 60,000 Determine (a) The inventory turnover and (b) The number of days sales in inventory. Round to one decimal place
-
Discuss American History
-
Your firm has developed a new lithium ion battery polymer that could enhance the performance of lithion ion batteries. These batteries have applications in many markets including cellphones, laptops,...
-
Need help analyzing statistical data 1. ANOVA) True or false: If we assume a 95% confidence level, there is a significant difference in performance generally across all groups. 2. (t-test) True or...

Study smarter with the SolutionInn App


