What is the energy, in joules and in megaelectronvolts, associated with the decay of 238 U? The
Question:
What is the energy, in joules and in megaelectronvolts, associated with the decay of 238U?
![]()
The nuclidic (atomic) masses in atomic mass units (u) are from Table D.5 in Appendix D:
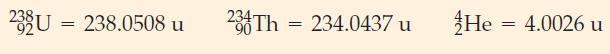
Transcribed Image Text:
233 U 92 234Th + He
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Analyze The key concept here is the fact that during a nuclear reaction a loss or gain of ma...View the full answer

Answered By

Munir Ahmed Jakhro
I am professional Tutor of of Business Courses, I did my four years Bachelor Degree from one of the Top Business schools of World "Institute of Business Administration" in year 2013. Since then I have been working as Tutor of Accounting, Finance tutor on different online platforms like this website. I am have experience of 6 years teaching business courses to students online and offline my professional job at national savings also helped me in accounting understanding .
4.90+
8+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(A) What is the energy associated with the decay of 146 Sm (145.913053 u) to 142 Nd (141.907719 u)? Use 4.002603 u as the mass of 4 He. (B) The decay of 222 Rn by -particle emission is accompanied...
-
(a) What is the energy in joules and electron volts of a photon of 420-nm violet light? (b) What is the maximum kinetic energy of electrons ejected from calcium by 420-nm violet light, given that the...
-
a. What is the energy in joules associated with photons that have a wavelength matching that of the color blue in the visible spectrum? b. Repeat part (a) for the color red. c. Do the results confirm...
-
What is data science, how does it differ from traditional statistics, explain data science process, including the key steps involve in it.
-
Arisael Company issued 20-year, $750,000 bonds with a stated rate of interest of 9%, compounded semiannually. The effective interest rate demanded by investors for bonds of this level of risk is also...
-
The rear window of a van is coated with a layer of ice at 0 C. The density of ice is 917 kg/m3. The driver of the van turns on the rear-window defroster, which operates at 12 V and 23 A. The...
-
Give three indicators of a multicollinearity problem.
-
Royal company is currently considering declaring a dividend to its common shareholders, according to one of the following plans: 1. Declare a cash dividend of $15 per share. 2. Declare a 10 percent...
-
The company is planning to make an investment in medical syringes and wants to sell one injector for $1. If the investment is made in zmir, the investment will be realized with a fixed cost of...
-
Write a plausible equation for the decay of tritium, 3 1 H, the radioactive isotope of hydrogen.
-
What proton number is found in a greater number of isotopes than any other proton number? What is the corresponding situation for neutron numbers?
-
Should the salvage value on equipment being replaced as part of a new project proposal be included as a cash inflow when calculating net present value and/or the internal rate of return for that...
-
Dr. Kovaleski is interested in examining whether quantity of sleep impacts problem solving ability. To test problem solving ability, the research team gave participants a puzzle and measured how long...
-
Can you please help me fill out the spreadsheet? Idexo Corporation is a privately held designer and manufacturer of licensed college apparel in Cincinnati, Ohio. In late 2020, after several years of...
-
CHECK FIGURE: Adjusted book balance = $2,837.06 Mae Telford, the controller of the Baylor Company, provided the following information: Bank statement balance Add: Baylor Company Bank Reconciliation...
-
Read the Scenario Congratulations, you are now the Police Chief in Anytown, USA. A city with 30,000 residents and you are responsible to provide 24 hour a day police coverage. You have a total of 45...
-
Here are summary statistics for randomly selected weights of newborn girls: n = 36, x = 3180.6 g, s = 700.5 g. Use a confidence level of 99% to complete parts (a) through (d) below. a. Identify the...
-
In problem 1-3, show that the indicated implication is true. 1. |x - 3| < 0.5 |5x - 15| < 2.5 2. |x + 2| < 0.3 |4x + 8| < 1.2 3. |x - 2| < /6 |6x - 12| <
-
Explain the Hawthorne effect.
-
You are provided with the following information for Web Inc. for the month ended June 30, 2010.Web uses the periodic method for inventory. Instructions(a) Calculate (i) ending inventory , (ii) cost...
-
You are provided with the following information for Mondello Inc. Mondello Inc. uses the periodic method of accounting for its inventory transactions. March 1 Beginning inventory 2,000 liters at a...
-
The management of Clare Co. asks your help in determining the comparative effects of the FIFO and LIFO inventory cost flow methods. For 2010, the accounting records show the following data....
-
1,600 Balance Sheet The following is a list (in random order) of KIP International Products Company's December 31, 2019, balance sheet accounts: Additional Paid-In Capital on Preferred Stock $2,000...
-
Question 3 4 pts 9 x + 3 x 9 if x 0 Find a) lim f(x), b) lim, f(x), C), lim , f(x) if they exist. 3 Edit View Insert Format Tools Table : 12pt M Paragraph B IV A2 Tv
-
Mr. Geoffrey Guo had a variety of transactions during the 2019 year. Determine the total taxable capital gains included in Mr. Guo's division B income. The transactions included: 1. On January 1,...

Study smarter with the SolutionInn App


