What must be the molarity of an aqueous solution of trimethylamine, (CH 3 ) 3 N, if
Question:
What must be the molarity of an aqueous solution of trimethylamine, (CH3)3N, if it has a pH = 11.12?
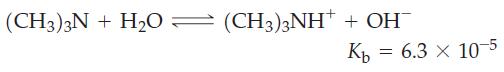
Transcribed Image Text:
(CH3)3 + H2O — (CH3)3NH' + OH Кь = 6.3 × 10-5
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
CH3 NHO CHNHOH Kb 63x10 and PH1112 According to HendersonHassalbalch ...View the full answer

Answered By

Shubhradeep Maity
I am an experienced and talented freelance writer passionate about creating high-quality content. I have over five years of experience working in the field and have collaborated with several renowned companies and clients in the SaaS industry.
At Herman LLC, an online collective of writers, I generated 1,000+ views on my content and created journal content for 100+ clients on finance topics. My efforts led to a 60% increase in customer engagement for finance clients through revamping website pages and email interaction.
Previously, at Gerhold, a data management platform using blockchain, I wrote and published over 50 articles on topics such as Business Finance, Scalability, and Financial Security. I managed four writing projects concurrently and increased the average salary per page from $4 to $7 in three months.
In my previous role at Bernier, I created content for 40+ clients within the finance industry, increasing sales by up to 40%.
I am an accomplished writer with a track record of delivering high-quality content on time and within budget. I am dedicated to helping my clients achieve their goals and providing exceptional results.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
What must be the molarity of an aqueous solution of NH 3 if it is 4.2% ionized?
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
What must be the molarity of an acetic acid solution if it has the same percent ionization as 0.100 M CH 3 CH 2 CO 2 H (propionic acid, K a = 1.3 x 10 -5 )?
-
On March 20, Harbor's petty cash fund of $100 is replenished when the fund contains $19 in cash and receipts for postage $40, supplies $26, and travel expense $15. Prepare the journal entry to record...
-
Raymond Manufacturing faces a liquidity crisisit needs a loan of $100,000 for 1 month. Having no source of additional unsecured borrowing, the firm must find a secured short-term lender. The firms...
-
Consider the following data for two variables, x and y. a. Compute the standardized residuals for these data. Do the data include any outliers? Explain. b. Plot the standardized residuals against...
-
1. You are valuing DistressCo, a company struggling to hold market share. The company currently generates $120 million in revenue but is expected to shrink to $100 million next year. Cost of sales...
-
Select a company you are interested in and obtain its annual reports by going to the companys website. Download the annual report for the most recent year. (On many companies websites, you will need...
-
A scenario exists that supports an argument in favor of a low dividend policy when: Group of answer choices corporate tax rates exceed personal tax rates. the majority of the stockholders have other...
-
What are [H 3 O + ], [OH - ], pH, and pOH of 0.55 M M HClO 2 ?
-
What mass of benzoic acid, C 6 H 5 COOH, would you dissolve in 350.0 mL of water to produce a solution with a pH = 2.85? C6H5COOH + HO = H0+ + CHCOO Ka = 6.3 x 10-5
-
For each of the following items, indicate whether a debit or a credit applies. A. Increase in retained earnings B. Decrease in prepaid rent C. Increase in dividends D. Decrease in salaries payable E....
-
Your friend Amber has approached you seeking advice concerning two investment opportunities that she is presently considering. Her classmate Simone has asked her for a loan of $5,000 to help...
-
Please read the following carefully. For each question on the exam, you should assume that: 1. unless expressly stated to the contrary, all events occurred in ?the current taxable year;? 2. all...
-
The pulse rates of 152 randomly selected adult males vary from a low of 37 bpm to a high of 117 bpm. Find the minimum sample size required to estimate the mean pulse rate of adult males. Assume that...
-
Can I get clear explanation how to work these. Thanking you in advance. 1. A rod 12.0 cm long is uniformly charged and has a total charge of -23.0 uC. Determine the magnitude and direction of the...
-
Poll Results in the Media USA Today provided results from a survey of 1144 Americans who were asked if they approve of Brett Kavanaugh as the choice for Supreme Court justice. 51% of the respondents...
-
Doris Angel asks, "Since stock dividends don't change anything, why declare them?" What is your answer to Doris?
-
9.Consider the reaction 3NO2(g)+H2O=2HNO3(aq)+NO(g) where Delta H=-137 kJ.How many kilojoules are released when 92.3g of NO2 reacts?
-
How are revenues and expenses reported on the income statement under (a) The cash basis of accounting and (b) The accrual basis of accounting?
-
Fees for services provided are billed to a customer during 2009. The customer remits the amount owed in 2010. During which year would the revenues be reported on the income statement under (a) The...
-
Employees performed services in 2009, but the wages were not paid until 2010. During which year would the wages expense be reported on the income statement under (a) The cash basis? (b) The accrual...
-
Summarize in your own words Sharps, Treynors, and Jensens Measures for assessing portfolio performance with respect to risk. Assess the portfolio performance of mutual fund VDIGX taking into...
-
Question 1 Slat and Company have recently set up a business which will manufacture and sell a furniture component, the F12 On the 19 August 2021, the company issued 85,000 of share capital for cash....
-
The following is Addison Corporations contribution format income statements for last month. The company has no beginning or ending inventories. A total of 10,000 units were produced and sold last...

Study smarter with the SolutionInn App


