How many milliliters of 0.250 M KMnO 4 are needed to react with 3.55 g of iron(II)
Question:
How many milliliters of 0.250 M KMnO4 are needed to react with 3.55 g of iron(II) sulfate, FeSO4? The reaction is as follows: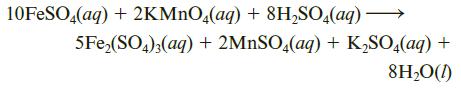
Transcribed Image Text:
10FESO,(aq) + 2KMNO,(aq) + 8H,SO,(aq) → 5Fe,(SO4);(aq) + 2MNSO,(aq) + K,SO,(aq) + 8H,O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
The reaction is 10FeSO4 2KMnO4 8HSO...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How many milliliters of 0.250 M KMnO4 are needed to react with 3.36 g of iron(II) sulfate, FeSO4? The reaction is as follows: 10FeSO4(aq) + 2KMnO4(aq) + 8H2SO4(aq) 5Fe2(SO4)3(aq) + 2MnSO4(aq) +...
-
For Reaction 1-7, how many milliliters of 0.165 0 M KMnO4 are needed to react with 108.0 mL of 0.1650 M oxalic acid? How many milliliters of 0.1650 M oxalic acid are required to react with 108.0 mL...
-
How many milliliters of 3.00 M H2SO4 are required to react with 4.35 g of solid containing 23.2 wt% Ba(NO3)2 if the reaction is Ba2++ SO24 BaSO4(s)?
-
In Exercises verify the identity. coshx = 1 + cosh 2x 2
-
One of the major measures of the quality of service provided by an organization is the speed with which the organization responds to customer complaints. A large family-held department store selling...
-
Write what you think is the best corporate policy for personal computer usage at work. LO.1
-
13. Use the information in Table 5. a. What is the price of a bond that pays one barrel of oil 2 years from now? b. What annual cash payment would the bond have to make in order to sell for $20.90?
-
James L. Skip Deupree, a developer, was building a development of townhouses called Point South in Destin, Florida. All the townhouses in the development were to have individual boat slips. Sam and...
-
Sanyu Sony started a new business and completed these transactions during December. Dec. 1 Sanyu Sony transferred $69,600 cash from a personal savings account to a checking account in the name of...
-
A plant supervisor must apportion her 40-hour workweek between hours working on the assembly line and hours supervising the work of others. She is paid $12 per hour for working and $15 per hour for...
-
How many milliliters of 0.250 M H 2 SO 4 (sulfuric acid) are required to react with 8.20 g of sodium hydrogen carbonate, NaHCO 3 , according to the following equation? H 2 SO 4 (aq) + 2NaHCO 3 (aq) h...
-
A 3.75-g sample of iron ore is transformed to a solution of iron(II) sulfate, FeSO 4 , and this solution is titrated with 0.150 M K 2 Cr 2 O 7 (potassium dichromate). If it requires 43.7 mL of...
-
Find the derivative of the function. y = (x 2 + 1) coth x/3
-
1) Factor the following Expressions (Write your factors only, don't show your work) a) 2x - 32 = c) 3x-2x-8= b) 2x-6x-8=
-
Bloomfield Inc. manufactures widgets. A major piece of equipment used to make the widget is nearing the end of its useful life. The company is trying to decide whether they should lease new equipment...
-
1. a. What is network management? Illustrate network management functional flowchart. [2.5] b. What encoding and decoding mechanisms are used in fast Ethernet and gigabit Ethernet? What is meant by...
-
Project Data: Sam Parker owns and operates a consulting firm called Business Solutions. The business began operating in October 202X. Transactions for October and November 202X have been recorded and...
-
3. Use Hooke's law to predict which one out of each pair vibrates at a higher wavenumber. Explain your answer. (7 points) a) C-H and C-D* b) C-C and C=C where: 1 k v = 2, v=wavenumber c = velocity of...
-
A detergent solution has a pH of 11.80 at 25C. What is the hydroxide-ion concentration?
-
The Cholesterol Level data sets give cholesterol levels of heart attack patients. Cholesterol measures are taken 2, 4, and 14 days aft er a patient has suffered a heart attack. Is there a significant...
-
A 250-g sample of water at 20.0oC is placed in a freezer that is held at a constant temperature of 20.0oC. Considering the water as the system, answer the following questions: a. What is the sign of...
-
A 20.0-g block of iron at 50.0oC and a 20.0 g block of aluminum at 45oC are placed in contact with each other. Assume that heat is only transferred between the two blocks. a. Draw an arrow indicating...
-
What is the enthalpy change for the preparation of one mole of liquid water from the elements, given the following equations? H2(g) +-02(g)--H20(g):AH, H20(1)--H20(g); ,up
-
4. The risk-free rate of return is 3.78% and the market risk premium is 6.42%. What is the expected rate of return on a stock with a beta of 1.09?
-
Maddox Resources has credit sales of $ 1 8 0 , 0 0 0 yearly with credit terms of net 3 0 days, which is also the average collection period. Maddox does not offer a discount for early payment, so its...
-
Selk Steel Co., which began operations on January 4, 2017, had the following subsequent transactions and events in its long-term investments. 2017 Jan. 5 Selk purchased 50,000 shares (25% of total)...

Study smarter with the SolutionInn App


