The element X below could be a. Sulfur b. Iodine c. Aluminum d. Phosphorus e. Silicon X.
Question:
The element X below could be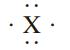
a. Sulfur
b. Iodine
c. Aluminum
d. Phosphorus
e. Silicon
Transcribed Image Text:
X.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 37% (8 reviews)
The ...View the full answer

Answered By

Amos Kiprotich
I am a wild researcher and I guarantee you a well written paper that is plagiarism free. I am a good time manager and hence you are assured that your paper will always be delivered a head of time. My services are cheap and the prices include a series of revisions, free referencing and formatting.
4.90+
15+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The thin outer layer of Earth, called the crust, contains only 0.50 percent of Earth's total mass and yet is the source of almost all the elements (the atmosphere provides elements such as oxygen,...
-
Bea Jones (age 32) moved from Texas to Florida in December 2013. She lives at 654 Ocean Way, Gulfport, FL 33707. Beas Social Security number is 466787359 and she is single. Her earnings and income...
-
Silicon is just below carbon in the periodic table. Predict the geometry of silicon tetrafluoride, SiF4.
-
In Exercises 7683, use a graphing utility to graph the function. Use the graph to determine whether the function has an inverse that is a function (that is, whether the function is one-to-one). f(x)...
-
On September 12, the cheapest-to-deliver bond on the December Treasury bond futures contract is the 9s of November YY+18. The bond pays interest semiannually on May 15 and November 15. Its price is...
-
Pick three consumer products of your choice. Illustrate the difficulties faced by the international marketer with respect to fluctuating exchange rates. Using the rationale presented in your chapter,...
-
The three groups of countries described in this chapter can be contrasted in terms of degree of economic freedom. It refers to the extent to which economic activities in a nation can take place...
-
Automobile unit sales at B. J. Scott Motors, Inc., provided the following 10-year time series: a. Construct a time series plot. Comment on the appropriateness of a linear trend. b. Using Excel Solver...
-
stock y has a beta of 1.33 and an expected return of 13.7 percent. stock z has a beta of 1.02 and an expected return of 11.4 percent. what would teh risk free rate have to be for the two stocks to be...
-
Suppose that the burning times of electric light bulbs approximate a normal curve with a mean of 1200 hours and a standard deviation of 120 hours. What proportion of lights burn for (a) Less than 960...
-
Which of the following represent configurations of indium ions in compounds? Explain your decision in each case. a. In 4+ [Kr]4d 9 b. In + [Kr]4d 10 5s 2 c. In 3+ [Kr]4d 10 d. In [Kr]4d 10 5s 1
-
Which of the following is the ground-state electron configuration of a C 3- ion? a. 1s 2 2s 2 2p 4 b. [He]2s 2 2p 6 c. [He]2s 1 d. 1s 2 2s 2 2p 5 e. 1s 2 2s 2 2p 6 3s 1
-
The following data were adapted from the financial statements of Kmart Corporation, prior to its filing for bankruptcy: a. Prepare a horizontal analysis for the income statement showing the amount...
-
Pacifico Company, a U . S . - based importer of beer and wine, purchased 1 , 7 0 0 cases of Oktoberfest - style beer from a German supplier for 4 5 9 , 0 0 0 euros. Relevant U . S . dollar exchange...
-
Consider each of the following scenarios and identify a behavioral intervention to address each issue in family work. A teenager not complying with curfew. One member of the couple not picking up...
-
Sandy Crane Hospital expanded its maternity ward to add patient rooms for extended hospital stays. They negotiated a 15-year loan with monthly payments and a large sum of $250,000 due at the end of...
-
2 (39 marks) R QUESTION 2 (39 marks) Roundworm Ltd is a group of companies with a 31 December year-end. The Roundworm group financial statements for the years 20.21 and 20.22 are given below:...
-
Vino Veritas Company, a U.S.-based importer of wines and spirits, placed an order with a French supplier for 1,400 cases of wine at a price of 240 euros per case. The total purchase price is 336,000...
-
Steam is accelerated by a nozzle steadily from a low velocity to a velocity of 280 m/s at a rate of 2.5 kg/s. If the temperature and pressure of the steam at the nozzle exit are 400C and 2 MPa, the...
-
Fill in each blank so that the resulting statement is true. A solution to a system of linear equations in two variables is an ordered pair that__________ .
-
An antiseptic solution contains hydrogen peroxide, H2O2, in water. The solution is 0.600 m H2O2. What is the mole fraction of hydrogen peroxide?
-
Concentrated hydrochloric acid contains 1.00 mol HCl dissolved in 3.31 mol H2O. What is the mole fraction of HCl in concentrated hydrochloric acid? What is the molal concentration of HCl?
-
Concentrated aqueous ammonia contains 1.00 mol NH3 dissolved in 2.44 mol H2O. What is the mole fraction of NH3 in concentrated aqueous ammonia? What is the molal concentration of NH3?
-
Phantom Consulting Inc. is a small computer consulting business. The company is organized as a corporation and provides consulting services, computer system installations, and custom program...
-
Sam owns a 25% in Spade, LLC. In 2021, Spade reports $100,000 or ordinary income. What is Sams qualified business income (QBI) deduction? answer is 5,000 but please show how to get it
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...

Study smarter with the SolutionInn App


