Use the solubility rules (Table 4.1) to decide which of the following compounds are expected to be
Question:
Use the solubility rules (Table 4.1) to decide which of the following compounds are expected to be soluble and which insoluble.
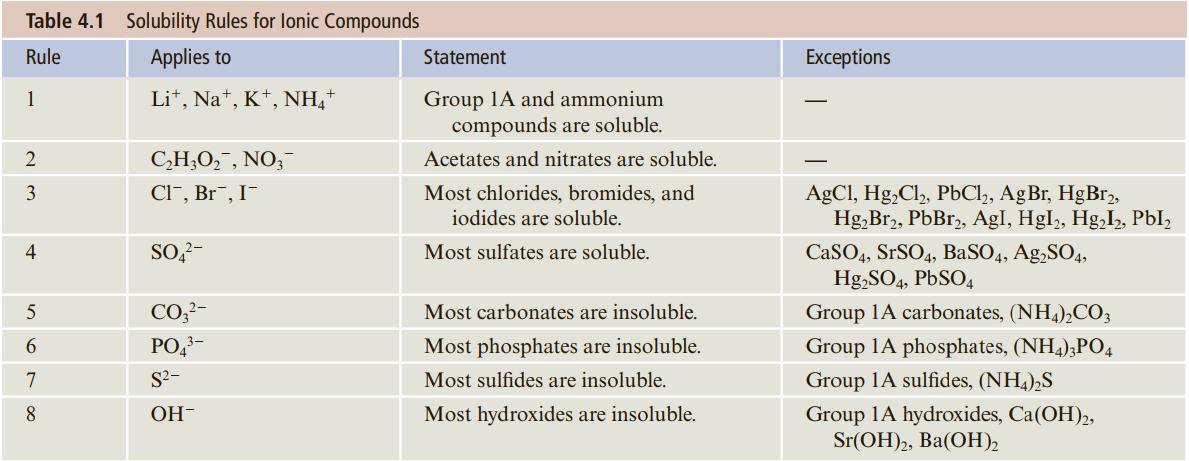
a. Mg(C2H3O2)2
b. NiS
c. Cr(NO3)2
d. Ca3(PO4)2
Transcribed Image Text:
Table 4.1 Solubility Rules for lonic Compounds Rule Applies to Statement Exceptions Lit, Na*, K*, NH," Group 1A and ammonium compounds are soluble. 1 C,H;O,, NO,- Acetates and nitrates are soluble. 3 CI", Br", I- Most chlorides, bromides, and AgCl, Hg,Cl,, PbCl,, AgBr, HgBr, Hg,Br2, PbBr,, AgI, Hgl, Hg,I, PbI, iodides are soluble. SO,?- CaSO4, SrSO4, BaSO4, Ag,SO4, Hg,SO4, PBSO4 4 Most sulfates are soluble. 5 CO,?- Most carbonates are insoluble. Group 1A carbonates, (NH4),CO; 6 PO,- Most phosphates are insoluble. Group 1A phosphates, (NH4),PO4 7 S2- Most sulfides are insoluble. Group 1A sulfides, (NH,),S 8 OH- Most hydroxides are insoluble. Group 1A hydroxides, Ca(OH),, Sr(ОH)2, Ba(ОН).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
a MgC 2 H 3 O 2 2 is soluble acetates are solub...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of the following compounds are aromatic? a. b. c. Cycloheptatrienyl cation d. e. f. g. Cyclononatetraenyl anion h. CH2=CHCH=CHCH=CH2
-
Which of the following compounds are chiral? (a) 2-Methylheptane (b) 3-Methylheptane (c) 4-Methylheptane (d) 1,1-Dibromopropane (e) 1,2-Dibromopropane (f) 1,3-Dibromopropane (g) Ethene, H2C=CH2 (h)...
-
Which of the following compounds are chiral? Draw each compound in its most symmetric conformation, star (*) any asymmetric carbon atoms, and draw any mirror planes. Label any meso compounds. You may...
-
Find the minimum and maximum values of the function subject to the given constraint. f(x, y) =xy, 4x +9y = 32
-
The study of whether birth decade can predict the number of software millionaires born in the decade. The data are reproduced in the table shown below. a. Construct a 95% confidence interval for the...
-
Define the concept of 'talent!
-
There is no difference between freshman class sizes for Pennsylvania colleges and California colleges.
-
Is the goal of Six Sigma quality realistic for services such as Blockbuster Video Stores or Redbox DVD Kiosks?
-
QUESTION 2 The subject property is a warehouse that is 5 0 feet by 2 0 0 feet by 1 6 feet high. The warehouse is cement block faced with brick on a concrete slab and was built in 1 9 8 0 for $ 7 5...
-
1. Given the risk, what would motivate an investor to purchase stock in Gogo? 2. Why would Gogo sell stock rather than taking on additional debt financing? Do you think that this was a good decision?...
-
You are given a solution of the ions Mg 2+ , Ca 2+ , and Ba 2+ . Devise a scheme to separate these ions using sodium sulfate. Note that magnesium sulfate is soluble.
-
The solubility of cobalt(II) iodate in water is 1.2 g/100 mL. Calculate the solubility product constant for cobalt(II) iodate, Co(IO 3 ) 2 .
-
The ledger of Swann Company contains the following balances: D. Swann, Capital $30,000; D. Swann, Drawing $2,000; Service Revenue $50,000; Salaries Expense $27,000; and Supplies Expense $4,000....
-
If the dose rate from a sample of Ga-67 is 0.052 mSv per hour at a distance of 1.1 m, then what would be dose rate at 3.5 m ?
-
A 1.6x10^9 p/s point source of Po210-Be source of 4.5 MeV is stored behind a X cm of paraffin, the dose equivalent rate is not to exceed 0.10 mSvh-1h at a distance of 1m. What is the X cm needed to...
-
X 10 Let A = -9 y 7 4 Z 210 If the kernel of A contains the vector what are x, y, and z? -2
-
8-22. E.O.Q., Carrying cost = Storing cost + Interest. Following data are available with respect to a certain material. Annual requirement.......... Cost to place an order.. Annual interest rate. _...
-
A new company started production. Job 1 was completed, and Job 2 remains in production. Here is the information from the job cost sheets from their first and only jobs so far: Job 1 Hours Total Cost...
-
What is the difference between pound-mass and pound-force?
-
The Home Depot is the leading retailer in the home improvement industry and one of the 10largest retailers in the United States. The company included the following on its January 29, 2012, balance...
-
Carbon monoxide and hydrogen react in the presence of a catalyst to form methanol, CH3OH: An equilibrium mixture of these three substances is suddenly compressed so that the concentrations of all...
-
A and B react to produce C according to the following chemical equation: A+ B C Amounts of A and B are added to an equilibrium reaction mixture of A, B, and C such that when equilibrium is again...
-
Given the hypothetical exothermic reaction A2(g) + 2B(g) 2AB(g) at equilibrium, decide which of the following containers represents the reaction mixture at the higher temperature? (The other...
-
Carnes Cosmetics Co.'s stock price is $58, and it recently paid a $2.50 dividend. This dividend is expected to grow by 21% for the next 3 years, then grow forever at a constant rate, g; and r s =...
-
You are the digital marketing director for High West fashions, a regional clothing company that specializes in custom t-shirts. Your company has decided to launch an online advertising campaign that...
-
In-the-money put options will automatically get exercised at the expiration. True OR False

Study smarter with the SolutionInn App


