A process for making small amounts of hydrogen by cracking ammonia is being considered, and residual uncracked
Question:
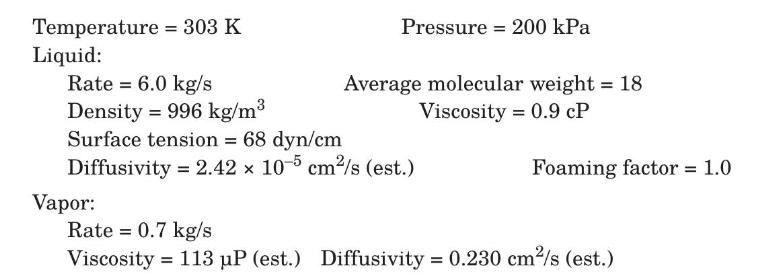
A process for making small amounts of hydrogen by cracking ammonia is being considered, and residual uncracked ammonia is to be removed from the resulting gas. The gas will consist of $\mathrm{H}_{2}$ and $\mathrm{N}_{2}$ in the molar ratio 3:1, containing $3 \% \mathrm{NH}_{3}$ by volume. The ammonia will be removed by scrubbing the gas countercurrently with pure liquid water in a sieve-tray tower. Conditions at the bottom of the tower are
For dilute solutions, $\mathrm{NH}_{3}-\mathrm{H}_{2} \mathrm{O}$ follows Henry's law, and at $303 \mathrm{~K}$ the slope of the equilibrium curve is $m=0.85$ (Treybal, 1980).
(a) Design a suitable cross-flow sieve tray for such a tower. Take $d_{o}=4.75 \mathrm{~mm}$ on an equilateral-triangular pitch $12.5 \mathrm{~mm}$ between hole centers, punched in stainless-steel sheet metal $2 \mathrm{~mm}$ thick. Use a weir height of $40 \mathrm{~mm}$. Design for an $80 \%$ approach to the flood velocity. Report details respecting tower diameter, tray spacing, weir length, gas-pressure drop, and entrainment in the gas. Check for excessive weeping.
(b) Estimate the tray efficiency corrected for entrainment for the design reported in part (a).
Step by Step Answer:






