An open kettle contains (50 mathrm{kmol}) of a dilute aqueous solution of methanol ( (2 mathrm{~mol} %)
Question:
An open kettle contains \(50 \mathrm{kmol}\) of a dilute aqueous solution of methanol ( \(2 \mathrm{~mol} \%\) of methanol), at the bubble point, into which steam is continuously sparged. The entering steam agitates the kettle contents so that they are always of uniform composition, and the vapor produced, always in equilibrium with the liquid, is led away. Operation is adiabatic. For the concentrations encountered, it may be assumed that the enthalpy of the steam and evolved vapor are the same, the enthalpy of the liquid in the kettle is essentially constant, and the relative volatility is constant at 7.6.
a) Show that, under these conditions:
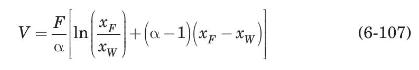
where \(V=\) mol of steam required.
b) Compute the quantity of steam to be introduced in order to reduce the concentration of methanol to \(0.1 \mathrm{~mol} \%\).
Answer: \(20.53 \mathrm{kmol}\)
Step by Step Answer:






