As shown in Problem 3.24, scrubbing of hydrogen sulfide from natural gas using water is not practical
Question:
As shown in Problem 3.24, scrubbing of hydrogen sulfide from natural gas using water is not practical since it requires large amounts of water due to the low solubility of $\mathrm{H}_{2} \mathrm{~S}$ in water. If a $2 \mathrm{~N}$ solution of monoethanolamine (MEA) in water is used as the absorbent, however, the required liquid flow rate is reduced dramatically because the MEA reacts with the absorbed $\mathrm{H}_{2} \mathrm{~S}$ in the liquid phase, effectively increasing its solubility.
For this solution strength and a temperature of $298 \mathrm{~K}$, the solubility of $\mathrm{H}_{2} \mathrm{~S}$ can be approximated by (de Nevers, 2000)
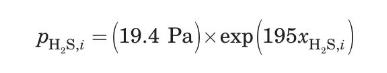
Repeat the calculations of Problem 3.24 but using a $2 \mathrm{~N}$ monoethanolamine solution as absorbent.
Data From Problem 3.24:-
A scheme for the removal of $\mathrm{H}_{2} \mathrm{~S}$ from a flow of $1.0 \mathrm{std} \mathrm{m}^{3} / \mathrm{s}$ of natural gas by scrubbing with water at $298 \mathrm{~K}$ and 10 atm is being considered. The initial composition of the feed gas is $2.5 \mathrm{~mol} \% \mathrm{H}_{2} \mathrm{~S}$. A final gas stream containing only $0.1 \mathrm{~mol} \%$
$\mathrm{H}_{2} \mathrm{~S}$ is desired. The absorbing water will enter the system free of $\mathrm{H}_{2} \mathrm{~S}$. At the given temperature and pressure, the system will follow Henry's law, according to $Y_{i}=48.3 X_{i}$, where $X_{i}=\mathrm{mol} \mathrm{H}_{2} \mathrm{~S} / \mathrm{mol}$ of water; $Y_{i}=\mathrm{mol} \mathrm{H}_{2} \mathrm{~S} / \mathrm{mol}$ of air.
(a) For a countercurrent absorber, determine the flow rate of water that is required if 1.5 times the minimum flow rate is used.
(b) Determine the composition of the exiting liquid.
(c) Calculate the number of ideal stages required.
Step by Step Answer:






