Consider the behavior of the amino acid alanine as a function of $mathrm{pH}$ shown in the following
Question:
Consider the behavior of the amino acid alanine as a function of $\mathrm{pH}$ shown in the following sequence of reactions.
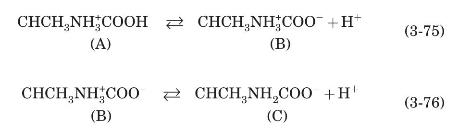
The sequence shown starts at low $\mathrm{pH}$ (high hydrogen ion concentration) where alanine has a positive charge. Using equilibrium constants $K_{a 1}$ and $K_{a 2}$, the coupled set of equations that result is (with the chemical species denoted as $\mathrm{A}, \mathrm{B}$, and $\mathrm{C}$ as illustrated above)
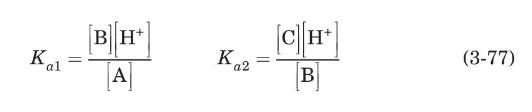
Using $[\mathrm{B}]_{0}$ to represent the initial molarity of the uncharged amino acid at neutral $\mathrm{pH}$, we have
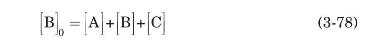
Since the two equilibrium constants for amino acids are so different (for alanine, for example, $\mathrm{p} K_{a 1}=2.3, \mathrm{p} K_{a 2}=9.9$ ), it can be assumed that at low $\mathrm{pH}$ species $\mathrm{C}$ will not be present, while at high $\mathrm{pH}$ species A will not be present.
(a) Show that under these circumstances
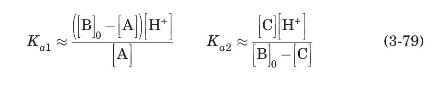
(b) Show that
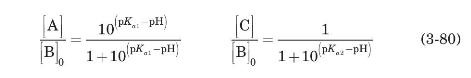
c) The average charge of an amino acid as a function of $\mathrm{pH}, f(\mathrm{pH})$, is given by
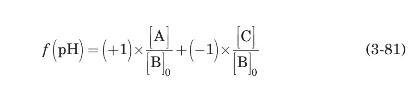
Combining equations (3-80) and (3-81) with the definition of the isoelectric point, show that, in this case,

Step by Step Answer:






