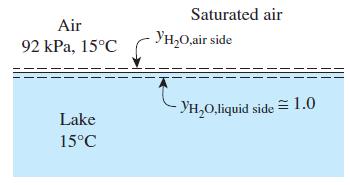
Determine the mole fraction of the water vapor at the surface of a lake whose temperature is
Question:
Determine the mole fraction of the water vapor at the surface of a lake whose temperature is 15°C and compare it to the mole fraction of water in the lake. Take the atmospheric pressure at lake level to be 92 kPa.
Transcribed Image Text:
Air 92 kPa, 15°C Lake 15°C Saturated air YH₂O,air side -YH₂O,liquid side = 1.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
To determine the mole fraction of water vapor at the surface of the lake we can use the concept of p...View the full answer

Answered By

Wonder Dzidzormenu
As a professional accountant and a teacher, I explain account ing concepts in a more practical way that makes students more connected to the subject.
With over 10 years of teaching accounting , I offer a well constructed , easily understood and in-depth explanations to students questions.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Heat And Mass Transfer Fundamentals And Applications
ISBN: 9780073398181
5th Edition
Authors: Yunus Cengel, Afshin Ghajar
Question Posted:
Students also viewed these Engineering questions
-
Determine the mole fraction of the water vapor at the surface of a lake whose surface temperature is 60F, and compare it to the mole fraction of water in the lake, which is very nearly 1.0. The air...
-
Determine the mole fraction of the water vapor at the surface of a lake whose temperature at the surface is 70F, and compare it to the mole fraction of water in the lake. Take the atmospheric...
-
Determine the mole fraction of dry air at the surface of a lake whose temperature is 18C. The air at the lake surface is saturated, and the atmospheric pressure at lake level can be taken to be 100...
-
In what respects did certain employees regard the University of Baths culture under Breakwell as falling short of the aspirations described in the 2016-21 strategic plan?
-
Presented below are the purchases and cash payments journals for Rosalez Co. for its first month of operations. In addition, the following transactions have not been journalized for July. The cost of...
-
Use the data in Exercise 6-5 to prepare comparative income statements for the month of January for the company similar to those shown in Exhibit 6.8 for the four inventory methods. Assume expenses...
-
Define trading account and state the objectives of preparing it.
-
Thirty patients who check out of the Rock Creek County Regional Hospital each week are asked to complete a questionnaire about hospital service. Since patients do not feel well when they are in the...
-
urrently, Meyers Manufacturing Enterprises (MME) has a capital structure consisting of 35% debt and 65% equity. MME's debt currently has a 6.8% yield to maturity. The risk-free rate (rRF) is 4.8%,...
-
Some critics argue that having different organizations establish accounting principles is wasteful and inefficient. Instead of mandating accounting standards, each company could voluntarily disclose...
-
Repeat Prob. 14154 for a water bath temperature of 55C. Data from problem 154 A glass bottle washing facility uses a well agitated hot water bath at 50C with an open top that is placed on the ground....
-
A glass bottle washing facility uses a well agitated hot water bath at 50C with an open top that is placed on the ground. The bathtub is 1 m high, 2 m wide, and 4 m long and is made of sheet metal so...
-
In Problem factor completely. 6x 2 + 67x + 35
-
Lucy is using a one-sample test based on a simple random sample of size = 24 to test the null hypothesis = 23.000 cm against the alternative hypothesis < 23.000 cm. The sample has mean 22.917 cm and...
-
A motorcyclist of mass 60 kg rides a bike of mass 40 kg. As she sets off from the lights, the forward force on the bike is 200N. Assuming the resultant force on the bike remains constant, calculate...
-
A load downward load P = 400 N is applied at B. It is supported by two truss members with member BA at an angle of 0 = 45 from horizontal and member BC at an 01 = angle of 02 25 from vertical....
-
Gross profit, defined as Net sales less Cost of products sold increased by $279 million in 2017 from 2016 and decreased by $2 million in 2016 from 2015. As a percent of sales, gross profit was 38.8%...
-
An electro-magnetic shield is to be made of galvanized steel with conductivity = 1.74 x 106 S/m, and magnetic permeability HR = 80. The thickness of cold rolled steel is in the following table. Gauge...
-
Caribou County Service Station started a customer loyalty program at the beginning of 2016 in which customers making cash purchases of gasoline at the gas bar are issued rewards in the form of...
-
What are current assets and current liabilities? How are they different from non-current assets and non-current liabilities?
-
Refer to the Computers & Security (July 2013) study of security issues with Microsoft products, Exercise 2.4. Recall that Microsoft periodically issues a Security Bulletin that reports the software...
-
Refer to the Environmental & Engineering Geoscience (Nov. 2012) study of settlement of shallow foundations on cohesive soil, Exercise 7.50. Recall that the researchers sampled 13 structures built on...
-
Each year the National Highway Traffic Safety Administration (NHTSA) conducts crash tests for new cars. Crash-test dummies are placed in the drivers seat and front passengers seat of a new-car model,...
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


