Hydrogen gas is used in a process to manufacture a sheet material of 6-mm thickness. At the
Question:
Hydrogen gas is used in a process to manufacture a sheet material of 6-mm thickness. At the end of the process, \(\mathrm{H}_{2}\) remains in solution in the material with a uniform concentration of \(320 \mathrm{kmol} / \mathrm{m}^{3}\). To remove \(\mathrm{H}_{2}\) from the material, both surfaces of the sheet are exposed to an airstream at \(555 \mathrm{~K}\) and a total pressure of \(3 \mathrm{~atm}\). Due to contamination, the hydrogen partial pressure is \(0.1 \mathrm{~atm}\) in the airstream, which provides a convection mass transfer coefficient of \(1.5 \mathrm{~m} / \mathrm{h}\). The mass diffusivity and solubility of hydrogen (A) in the sheet material (B) are \(D_{\mathrm{AB}}=2.6 \times 10^{-8} \mathrm{~m}^{2} / \mathrm{s}\) and \(S_{\mathrm{AB}}=160 \mathrm{kmol} / \mathrm{m}^{3} \cdot \mathrm{atm}\), respectively.
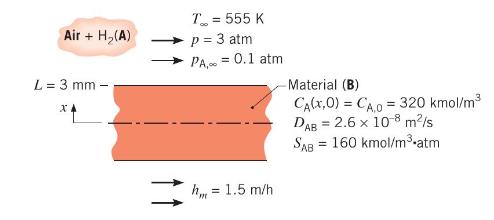
(a) If the sheet material is left exposed to the airstream for a long time, determine the final content of hydrogen in the material \(\left(\mathrm{kg} / \mathrm{m}^{3}\right)\).
(b) Identify and evaluate the parameter that can be used to determine whether the transient mass diffusion process in the sheet can be assumed to be characterized by a uniform concentration at any time during the process. Hint: This situation is analogous to that used to determine the validity of the lumped-capacitance method for a transient heat transfer analysis.
(c) Determine the time required to reduce the hydrogen mass density at the center of the sheet to twice the limiting value calculated in part (a).
Step by Step Answer:

Fundamentals Of Heat And Mass Transfer
ISBN: 9781119220442
8th Edition
Authors: Theodore L. Bergman, Adrienne S. Lavine





