Methanol ( = 791 kg/m 3 and M = 32 kg/kmol) undergoes evaporation in a vertical tube
Question:
Methanol (ρ = 791 kg/m3 and M = 32 kg/kmol) undergoes evaporation in a vertical tube with a uniform cross-sectional area of 0.8 cm2. At the top of the tube, the methanol concentration is zero, and its surface is 30 cm from the top of the tube (Fig. P14–104). The methanol vapor pressure is 17 kPa, with a mass diffusivity of DAB = 0.162 cm2/s in air. The evaporation process is operated at 25°C and 1 atm.
(a) Determine the evaporation rate of the methanol in kg/h
(b) Plot the mole fraction of methanol vapor as a function of the tube height, from the methanol surface (x = 0) to the top of the tube (x = L).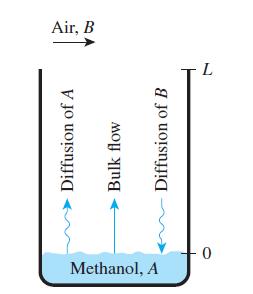
Step by Step Answer:
Related Book For 

Heat And Mass Transfer Fundamentals And Applications
ISBN: 9780073398181
5th Edition
Authors: Yunus Cengel, Afshin Ghajar
Question Posted:




