Repeat Example 3.1, but for a liquid concentration of $0.5 mathrm{~mol}$ fraction of benzene and a temperature
Question:
Repeat Example 3.1, but for a liquid concentration of $0.5 \mathrm{~mol}$ fraction of benzene and a temperature of $310 \mathrm{~K}$.
Data From Example 3.1:-
Raoult’s law may be used to determine phase compositions for the binary system of benzene and toluene, at low temperatures and pressures. Determine the composition of the vapor in equilibrium with a liquid containing 0.4 mol fraction of benzene at 300 K and calculate the total equilibrium pressure. Estimate the vapor pressure of benzene and toluene at 300 K from the Antoine equation,
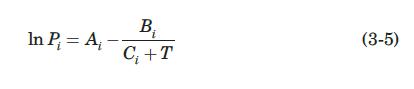
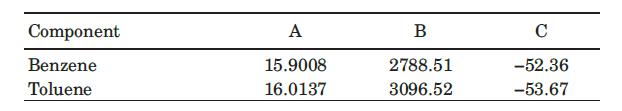
where Pi is the vapor pressure of component i, in mm Hg, and T is the temperature in K. The Antoine constants for benzene and toluene are given in the following table (Himmelblau, 1989):
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





