When a salt dissociates in solution, ions rather than molecules diffuse. In the absence of an electric
Question:
When a salt dissociates in solution, ions rather than molecules diffuse. In the absence of an electric potential, the diffusion of a single salt may be treated as molecular diffusion. For dilute solutions of a single salt, the diffusion coefficient is given by the Nernst-Haskell equation (Harned and Owen, 1950):
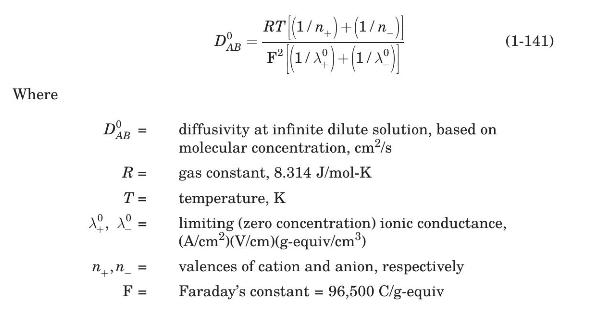
Values of limiting ionic conductances for some ionic species at $298 \mathrm{~K}$ are included in Table 1.4. If conductance values at other temperatures are needed, an approximate correction factor is $T /\left(334 \mu_{W}\right)$, where $\mu_{W}$ is the viscosity of water at $T$, in $\mathrm{cP}$.
(a) Estimate the diffusion coefficient at $298 \mathrm{~K}$ for a very dilute solution of $\mathrm{HCl}$ in water.
(b) Estimate the diffusion coefficient at $273 \mathrm{~K}$ for a very dilute solution of $\mathrm{CuSO}_{4}$ in water. The viscosity of liquid water at $273 \mathrm{~K}$ is $1.79 \mathrm{cP}$.
Data From Table 1.4:-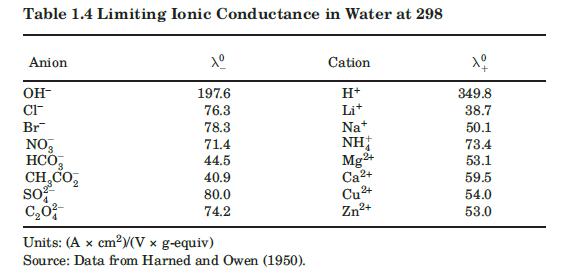
Step by Step Answer:






