(a) Given that U(0 K) and f H(298 K) for MgO are 3795 and 602 kJ...
Question:
(a) Given that ∆U(0 K) and ∆fH°(298 K) for MgO are −3795 and −602 kJ mol−1 respectively, derive a value for ∆EAH°(298 K) for the reaction:
![]()
Other data: see Appendices.
(b) Compare the calculated value with that obtained using electron affinity data from Appendix 9, and suggest reasons for any differences.
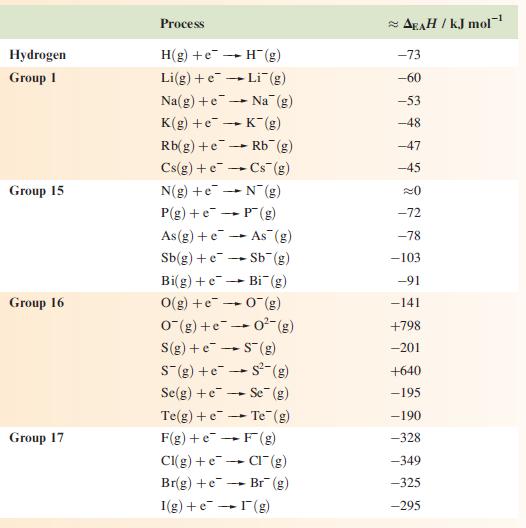
Appendix 9
Approximate enthalpy changes, ∆EAH(298 K), associated with the gain of one electron by a gaseous atom or anion. A negative enthalpy (∆H), but a positive electron affinity (EA), corresponds to an exothermic process. ∆EAH(298K) ≈ ∆U(0K) = −EA
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





