(a) Sr 2 RuH 6 crystallizes in a lattice that can be described in terms of the...
Question:
(a) Sr2RuH6 crystallizes in a lattice that can be described in terms of the CaF2 structure type with octahedral [RuH6]4− ions replacing Ca2+ ions, and Sr2+ ions replacing F− ions. Sketch a unit cell of CaF2. Show that in Sr2RuH6, each [RuH6]4− ion is surrounded by eight Sr2+ ions in a cubic array.
(b) Suggest products for the following reactions:
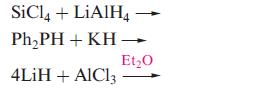
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





